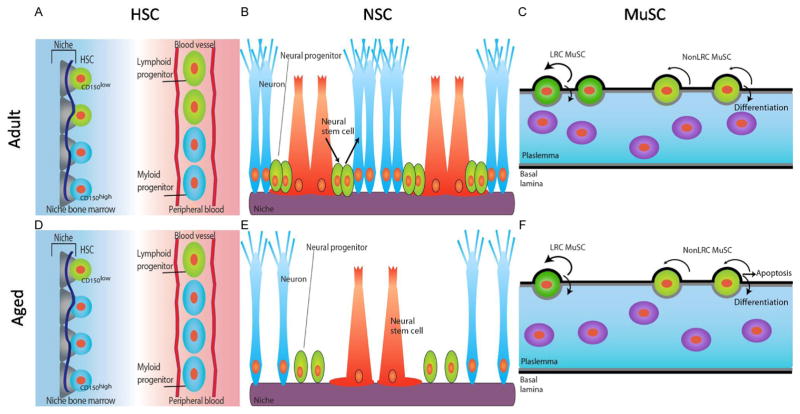
Figure 14.2.
Schematic diagrams of adult stem cells and niche in bone marrow, brain, and muscle tissue. (A, D) Quiescent adult hematopoietic stem cells (HSCs) are located in the endosteal region of adult (A) and aged (D) bone marrow (left) residing in a niche composed of endothelial cells, osteoblasts (gray), and perivascular cells (dark blue line). The adult HSC pool consists of lymphoid-biased (CD150low) HSCs (green) and myeloid-biased (CD150high) HSCs (blue), with high self-renewal potential and balanced differentiation toward lymphoid (green, elongated) and myeloid progenitor cells (blue, elongated) in the peripheral blood (right, A and D). In aged HSC niche, selective expansion/retention of myeloid-biased CD150high HSCs skewing differentiation toward the myeloid lineage due to the robust self-renewal potential of aged CD150high HSCs compared to CD150low HSCs. (B, E) Neural stem cells (NSCs) reside at subgranular zone (SGZ) of the dentate gyrus of the hippocampus in adult (B) and aged (E) brain. NSCs (red) can give rise to neural progenitors (green), which progressively differentiate into neurons (blue). Systemic factors from the blood and local niche factors (purple) control NSC number and function. With age, number of NSCs and adult neurogenesis (making new neurons) declines. (C, F) Muscle satellite stem cells (MuSCs) in adult (C) and aged (F) muscle fibers. MuSCs (green) reside between the plasmalemma (gray line) and the basal lamina (black line) of muscle fibers. Long-term label retaining (LRC) MuSCs (dark green) have a high capacity for self-renewal and differentiation into muscle. nonLRC MuSCs (light green) are capable of differentiation but have limited self-renewal capacity. With age, the number of LRC MuSCs and fusion competent progenitors declines leading to a reduced number of functional stem cells and defective muscle repair.