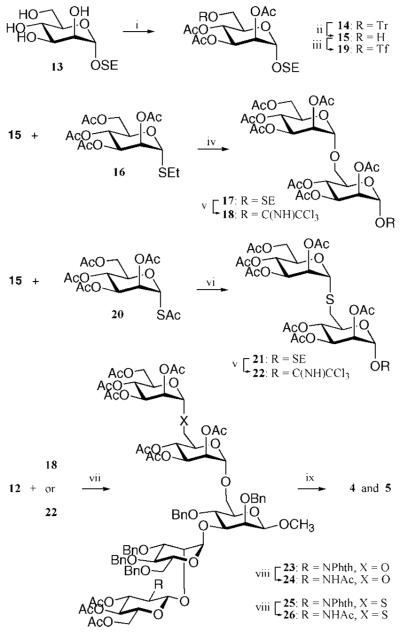
Scheme 2a.
a Reagents and conditions: (i) TrCl, pyridine, 80 °C, and then Ac2O, pyridine (96%); (ii) FeCl3 · 6H2O, DCM (82%); (iii) Tf2O, 2,6-lutidine, DCM, −40 °C; (iv) NIS, TfOH, DCM, 0 °C (76% for 17, 86% for 22); (v) diethylamine, DMF, 0 °C (73%); (vi) TFA, DCM, and then trichloroacetonitrile, DBU, DCM; (vii) TMSOTf, DCM, (80% for 23, 83% for 25); (viii) H2NNH2 · H2O, EtOH, 90 °C, and then Ac2O, pyridine; (ix) NaOMe, MeOH, and then Na (s), NH3 (l), THF, −78 °C (86% for 4, 87% for 5).