Scheme 3a.
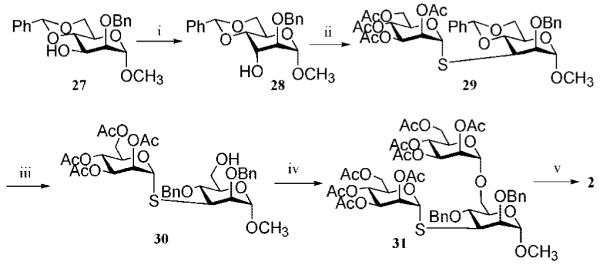
a Reagents and conditions: (i) DMSO, 1:2 Ac2O/DMSO, and then NaBH4, 1:1 DCM/MeOH (75%); (ii) Tf2O, 1:2 pyridine/DCM, and then 20, DMF, diethylamine, 0 °C (61%); (iii) BH3 in THF, Bu2BOTf (63%); (iv) 16, NIS, TfOH, DCM, 0 °C (77%); (v) NaOMe, MeOH, and then Na/NH3(l), −78 °C (70%).
