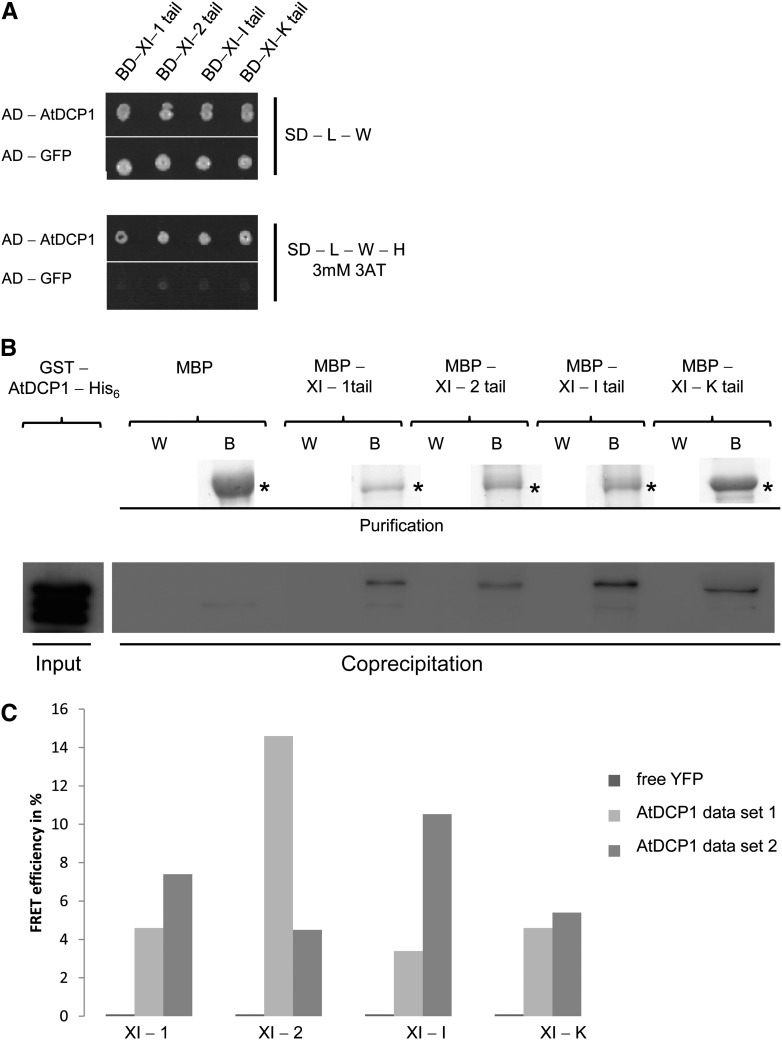
Figure 1.
The P-body protein AtDCP1 interacts directly with class XI myosins in Arabidopsis. A, Yeast two-hybrid interaction between AtDCP1 and myosin XI tails. Top, Double-transformed yeast cells on selective dropout (SD) medium lacking Leu (−L) and Trp (−W). Bottom, Interaction between AtDCP1 N-terminally fused to the GAL4-AD and the tails of XI-1, XI-2, XI-I, and XI-K, N-terminally fused to the GAL4-BD, on selective dropout medium lacking Leu, Trp, and His (−H), supplemented with 3 mm 3-aminotriazole (3AT). GFP N-terminally fused to the GAL4-AD was included as a negative control. B, Coprecipitation of AtDCP1 with all myosin XI tails investigated. Input of GST-AtDCP1-His6 (67 kD; 0.18 µg) per reaction was detected by anti-His6 antibody staining. Resin-bound MBP fusions (called B for beads) are shown by Coomassie Blue-stained gels. Coprecipitations of GST-AtDCP1-His6 with the tails of XI-1 (*, approximately 106 kD; 1.6 µg), XI-2 (*, approximately 104 kD; 1.7 µg), XI-I (*, approximately 105 kD; 2.0 µg), and XI-K (*, approximately 100 kD; 2.5 µg) were detected by anti-His6 antibody staining. The wash fraction (W) and bead fraction are presented. No coprecipitation was observed between MBP (*, approximately 42 kD; 2.8 µg) and GST-AtDCP1-His6. C, FRET efficiencies between CFP-tagged AtDCP1 and the YFP-tagged tails of XI-1, XI-2, XI-I, and XI-K in lysates of Arabidopsis cell suspension culture. No FRET could be detected for the negative control AtDCP1-CFP and free YFP. FRET efficiencies from two independent experiments are shown and represent the trend of at least four biological replicates per sample.