Abstract
Background
Little is known about the cost-effectiveness of external beam radiation therapy (EBRT) or newer radiation therapy (RT) modalities such as intensity modulated radiation (IMRT) or brachytherapy among older women with favorable-risk breast cancer.
Methods
Using a Markov model, we estimated the cost-effectiveness of no RT, EBRT, and IMRT over 10 years. We estimated the incremental cost-effectiveness ratio (ICER) of IMRT compared with EBRT under different scenarios to determine the necessary improvement in effectiveness for newer modalities to be cost-effective. We estimated model inputs using women in the Surveillance, Epidemiology, and End Results–Medicare database fulfilling the Cancer and Leukemia Group B C9343 trial criteria.
Results
The incremental cost of EBRT compared with no RT was $9500 with an ICER of $44600 per quality-adjusted life year (QALY) gained. The ICERs increased with age, ranging from $38300 (age 70–74 years) to $55800 (age 80 to 94 years) per QALY. The ICERs increased to more than $63800 per QALY for women aged 70 to 74 years with an expected 10-year survival of 25%. Reduction in local recurrence by IMRT compared with EBRT did not have a substantial impact on the ICER of IMRT. IMRT would have to increase the utility of baseline state by 20% to be cost-effective (<$100000 per QALY).
Conclusions
EBRT is cost-effective for older women with favorable risk breast cancer, but substantially less cost-effective for women with shorter expected survival. Newer RT modalities would have to be substantially more effective than existing therapies in improving quality of life to be cost-effective.
Although the use of external beam radiation therapy (EBRT) after breast-conserving surgery has been proven in clinical trials to improve local control and survival in women with early-stage breast cancer, this survival benefit has not been shown in an elderly population that typically has more indolent disease (1–8). In fact, current trial-based recommendations suggest that adjuvant radiation therapy (RT) may be omitted in a subgroup of older women with favorable-risk breast cancer (women aged >70 years with tumor size ≤2cm, estrogen receptor–positive status, and lymph node–negative disease) (9–11). Despite these guidelines, RT continues to be used in older women, raising concerns about overuse (12). It is important to consider the cost-effectiveness of RT, which has not yet been assessed among older women with early-stage breast cancer (13,14). It is also important to consider how changes in life expectancy affect the cost-effectiveness of RT, because life expectancy impacts the time at risk for recurrence and thus the effectiveness of RT in older women.
In addition to evaluating the cost-effectiveness of widely used and trial-tested treatments such as EBRT, newer and costlier technologies such as brachytherapy and intensity modulated RT (IMRT) are diffusing into clinical practice in the absence of comparative effectiveness data (15,16). A framework is needed to allow practitioners and policy makers to assess newer cancer treatments in the absence of substantial clinical data. To address this need, cost-effectiveness analysis can be used in a different manner, by first assessing existing trial-tested interventions (such as EBRT) and then integrating costs associated with newer modalities to determine how much more effective they would have to be to be incrementally cost-effective when compared with the standard of care.
Although clinical trials comparing brachytherapy to EBRT are ongoing (5,13,15–18), brachytherapy has not demonstrated any benefit on cancer control or overall or disease-free survival and may be inferior to EBRT in subsequent mastectomy rate and risk of complications (19,20). Although IMRT has demonstrated a reduction in toxicity and improved cosmesis, it remains substantially more expensive in the United States, and it is unclear how IMRT affects patient-reported quality of life (QoL) (21,22). Therefore, it is important to understand the balance between the costs and potential benefits of newer modalities.
We therefore estimated the cost-effectiveness of EBRT using Medicare expenditures to estimate total cancer-related costs. Second, we used survival data of older women to estimate the cost-effectiveness of EBRT across age and comorbidity groups. Finally, we explored the incremental costs for the newer RT modalities and projected how much more effective they would have to be relative to EBRT to be cost-effective.
Methods
Basic Model and Model Assumptions
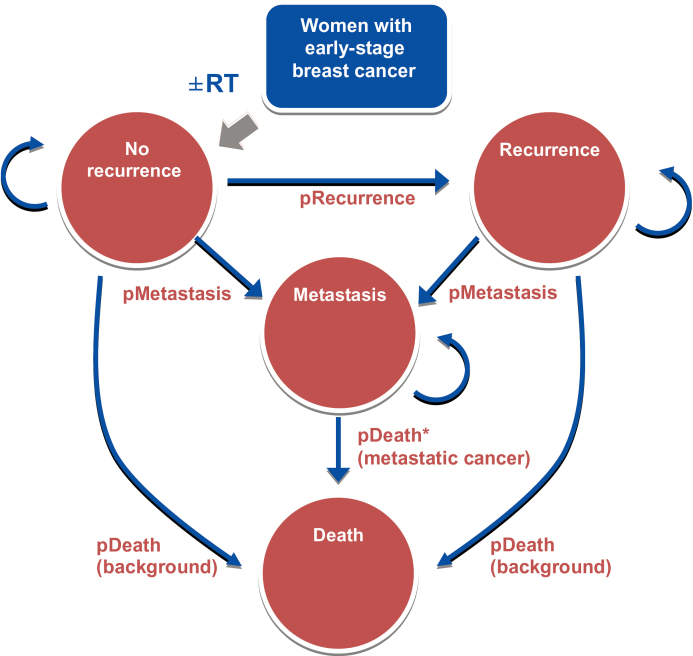
We designed a Markov model to simulate clinical outcomes, estimate quality-adjusted life-years (QALYs) gained, and determine the incremental cost-effectiveness ratio (ICER) of various RT modalities (EBRT, IMRT, brachytherapy) from a payer perspective over a 10-year horizon in older women with early-stage breast cancer. We compared the incremental costs and health benefits of these RT modalities. Acknowledging the limited effectiveness information for the newer modalities, we estimated the ICER of the newer modalities (IMRT and brachytherapy) compared with EBRT under different scenarios, assuming the newer modalities improved recurrence-free survival and/or utility. We then estimated the necessary improvement in effectiveness over EBRT for the newer modalities to be cost-effective based on two common willingness-to-pay thresholds of $50000 per QALY and $100000 per QALY (23–25). Three hypothetical cohorts of women starting at ages 70, 75, and 80 years were created to determine the effect of age. We assumed that all women are initially in a no-recurrence health state and subsequently transition to one of four health states (no recurrence, local recurrence, metastasis, or death) each year (Figure 1). We assumed that 1) survival was similar in no RT and RT groups; 2) RT reduced the risk of recurrence, according to the results derived from the Cancer and Leukemia Group B C9343 trial; and 3) the costs associated with various RT modalities were based on matched breast cancer case patients and non–breast cancer control subjects. We confined our analysis to 10 years because the efficacy measure (in terms of recurrence probability) was derived from C9343, which reported 10-year trial results. We used a payer perspective because decisions that influenced coverage and reimbursement were made by Medicare and our research question focused specifically on the costs and benefits of newer RT modalities among Medicare beneficiaries. All analyses were performed on TreeAge Pro 2012 (Williamstown, MA) and SAS version 9.2 (SAS Institute, Cary, NC). The Yale Human Investigation Committee determined that this study did not involve human subjects.
Figure 1.
Model overview. pDeath (background) = annual probability of death (refers to background mortality); pDeath* (metastatic cancer) = annual probability of death from metastatic breast cancer, including breast cancer specific mortality and background mortality; pMetastasis = annual probability of metastasis; pRecurrence = annual probability of recurrence; RT = radiation therapy.
Data Source and Sample Selection
We determined cost and overall survival using the Surveillance, Epidemiology, and End Results (SEER)–Medicare database, which links population-based cancer registries with Medicare claims data (26). The database also includes a 5% random sample of Medicare beneficiaries without cancer who reside in the SEER areas. We identified a sample of women who met the eligibility criteria for the C9343 trial (aged ≥70 years, tumor size ≤ 2cm, estrogen receptor–positive status, lymph node–negative disease), were diagnosed in the period from 1998 to 2007, and received breast-conserving surgery. We selected women who fulfilled C9343 criteria because the results from this trial shaped current recommendations stating that RT can be safely omitted in this population; thus our model parameters reflect characteristics of a population for whom there are distinct trial-based guidelines. We estimated 10-year survival in our SEER–Medicare sample according to age group in women who were diagnosed in the period from 1998 to 1999, for whom we had 10 years of follow-up data and determined an annual mortality rate. We used C9343 trial results to estimate probabilities for annual recurrence and metastasis. All mortality, recurrence, and metastasis rates were converted to annual transition probabilities as model inputs (Table 1). We validated our model-based estimates to the C9343 trial and SEER–Medicare in terms of a variety of outcomes (Supplementary Table 1, available online). We used C9343 data to estimate the effectiveness of RT in preventing a recurrence as well as the probability of metastatic disease. Details of our sample selection and model validation are described in the Supplementary Materials, which include both Supplementary Methods and Supplementary Tables 1 to 5 (available online).
Table 1.
Model input*
| Model assumptions | Value (range) | Source |
|---|---|---|
| Utilities, by age, treatment, and recurrence status | ||
| A. Utilities according to treatment and recurrence status† Conservative surgery and radiation therapy with no recurrence Conservative surgery and radiation therapy with isolated local recurrence Conservative surgery alone with no recurrence Conservative surgery alone with isolated local recurrence |
0.92 (0.05–1.00) 0.82 (0.00–0.99) 0.88 (0.00–1.00) 0.81 (0.00–0.99) |
Hayman et al. (30) |
| Distant metastases | 0.7 (0.6–0.8) | |
| B. Utility modifier according to age‡ 70–74 y 75–79 y 80–84 y >85 y |
0.716 0.675 0.623 0.59 |
Stout et al. (32) |
| Survival, all women† | SEER–Medicare | |
| 5-year survival | ||
| 70–74 y | 91.1% | |
| 75–79 y | 86.6% | |
| 80–94 y | 70.3% | |
| 10-year survival | ||
| 70–74 y | 73.6% (70.4–76.6) | |
| 75–79 y | 61.2% (57.4–64.8) | |
| 80–94 y | 33.4% (29.7–37.2) | |
| Annual recurrence probability, no RT† | 0.01 (0.007–0.016) | CALGB C9343 (11) |
| Relative risk of recurrence when receiving RT† | 0.18 (0.07–0.42) | CALGB C9343 (11) |
| Annual metastasis probability† | 0.005 (0.003–0.009) | CALGB C9343 (11) |
| Annual death probability from metastatic breast cancer* | 0.210–0.238§ | SEER (40) |
| Cancer-related costs per patient, mean (SD)† | SEER–Medicare | |
| No RT | $5593 (30895) | |
| EBRT | $15396 (26921) | |
| IMRT | $23605 (34337) | |
| Brachytherapy | $23628 (26833) | |
| Other costs | ||
| Recurrence, mastectomy,† mean (SD) | $6250 (4475) | SEER–Medicare |
| Metastatic care, mean | $37771 | Rao et al. (27) |
| Continued phase costs, mean | $284 (2–4 y) | SEER–Medicare |
| $212 (after year 4) | ||
| Death, last year of life, mean (SD)† | $44732 (48183) | SEER–Medicare |
| Annual discount rate, QALYs and costs | 0.03 (0–0.07) | |
* QALY= quality-adjusted life-year; RT= radiation therapy; SD = standard deviation.
† Included in the probabilistic sensitivity analysis.
‡ Multiply the age-specific utility by the relevant utility in section A. For example, the utility for a woman aged 73 years receiving RT without recurrence is 0.659 (0.92 x 0.716).
§ Estimates had been calibrated at 1-year incremental.
Cost and Utility Inputs
Each cancer patient was matched with a noncancer control subject based on age, race, comorbidity, region, and year of diagnosis (or year of randomly assigned index date for control subject). Total mean costs for initial treatment among no RT, EBRT, IMRT, and brachytherapy patients were calculated as all costs to Medicare (inpatient, outpatient facility, physician, home health, hospice, and Durable Medical Equipment claims) from a payer perspective in the 2 months before through 12 months after date of diagnosis/index date. Each cancer patient’s cancer-related cost was calculated as the difference between her total cost and that of her matched control subject (Table 1; Supplementary Methods, available online). We calculated the continuing phase cost per year, as well as end-of-life costs by calculating the costs in the last year of life. Cost for distant metastasis was based on prior literature (27). Costs were adjusted for inflation and geographic price differences to 2012 US dollars (28,29). We abstracted utility weights for each health state from the literature and then age-adjusted these utilities at 5-year increments using previously reported trends (30–32). Utilities varied based on age, receipt of RT, and metastatic and recurrence status. Costs and utilities were discounted at an annual rate of 3%.
Life Expectancy and Comorbidity Analysis
To assess the effect of age and comorbidity burden on the cost-effectiveness estimates, we constructed a separate noncancer sample by randomly selecting a subset of 50000 women aged 70 to 94 years between 1998 and 1999 to allow for 10-year follow-up. We categorized this noncancer sample by age and number of clinical comorbidities previously found to be associated with survival in noncancer patients (33). We chose to use survival data from a noncancer sample to allow for adequate sample size for each age and comorbidity combination. Using our noncancer sample, we determined the actual survival for each age and comorbidity combination (eg, patients aged 70–74 years with 1–2 comorbid conditions). Each combination of age and comorbidity was categorized into four groups based on 10-year survival (0%–25%, 25%–50%, 50%–75%, 75%–100%). We conducted a series of simulations integrating both age and mortality rate (assuming a constant mortality rate during 10 years) to estimate the range of cost-effectiveness of EBRT across these 10-year survival quartiles (ie, the range of ICERs for a predicted 10-year survival of <25%, 25%–50%, 50%–75%, >75%).
Sensitivity Analysis
We performed a series of one-way sensitivity analyses to determine the variability in the ICER as a function of the cost of RT, utility of RT, treated-recurrence probability, metastasis probability, and cost of recurrence. We conducted a two-way sensitivity analysis to investigate how much better the reduction in recurrence and improvement in age-specific QoL would need to be for the newer modalities to be cost-effective. We performed probabilistic sensitivity analysis to assess uncertainty and robustness by specifying distributions for model parameters following the standard Bayesian framework (Supplementary Methods, available online). When appropriate, we used beta distributions for probability parameters and utility estimates, log-normal distributions for relative risk parameters, and normal distribution for cost parameters (34). The distributions of input parameters were drawn 100000 times, and an acceptability curve was created.
Results
We included 18340 Medicare beneficiaries who met the C9343 eligibility criteria. The 10-year survival among all C9343 eligible women for whom we had 10-year follow-up data varied between 73.6% for women aged 70 to 74 years and 33.4% for women aged 80 to 94 years (Table 1). The 10-year probability of mastectomy-free survival for women receiving no RT in our SEER–Medicare sample was 96.4%, consistent with the C9343 trial (96%).
Our Markov model estimated the total costs for a 70-year old woman receiving EBRT during 10-years of follow-up to be approximately $29500, compared with $20077 for no RT, resulting in an incremental cost of approximately $9500 (Table 2). Using the SEER–Medicare population percentages by age as the weights, we calculated the cost-effectiveness of EBRT for all women in our sample to be $44600 per QALY. We estimated the QALYs experienced for a 70-year old woman to be 0.25 greater for EBRT than for no RT (5.42 and 5.17, respectively), resulting in an ICER for EBRT of $38300 per QALY. The ICERs for EBRT increased with increasing age, with an 80-year old woman experiencing 0.17 more QALYs with EBRT than no RT, corresponding to an ICER of $55800 per QALY.
Table 2.
Cost-effectiveness estimates for women with favorable early-stage breast cancer*
| Model result | Age, y | No RT | EBRT | Incremental changes | IMRT† | Incremental changes, IMRT† |
|---|---|---|---|---|---|---|
| Costs, $, per woman | 70–74 | 20077 | 29500 | 9423 | 37710 | 8209 |
| 75–79 | 24328 | 33774 | 9445 | 41983 | 8209 | |
| 80–94 | 34058 | 43569 | 9510 | 51778 | 8209 | |
| QALY per woman | 70–74 | 5.171 | 5.418 | 0.246 | 5.524 | 0.106 |
| 75–79 | 4.596 | 4.814 | 0.218 | 4.909 | 0.095 | |
| 80–94 | 3.608 | 3.778 | 0.170 | 3.852 | 0.074 | |
| Incremental cost-effectiveness ratio, $/QALY* |
70–74 | — | 38300 | 77100 | ||
| 75–79 | — | 43200 | 86700 | |||
| 80–94 | — | 55800 | 110500 | |||
| All | — | 44600 | 89300 |
* Minor discrepancies may exist because of rounding. EBRT= external beam radiation therapy; IMRT= intensity modulated radiation therapy; QALY=quality-adjusted life-year; RT= radiation therapy.
† Assuming that the recurrence rate is identical in the group receiving EBRT or IMRT and IMRT increases utility by 25% of the difference between utility receiving EBRT without recurrence and utility of healthy women without breast cancer. ICERs are rounded to the nearest hundred.
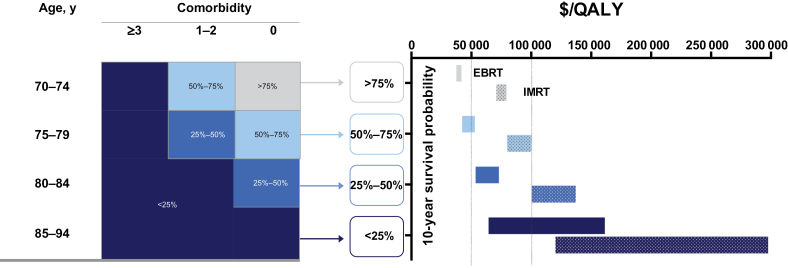
The cost-effectiveness for EBRT varied by age and comorbidity status (Figure 2). Older women with more comorbidity had a decreased 10-year survival probability, which corresponded to substantially less favorable cost-effectiveness for EBRT. Specifically, the ICER for EBRT was between $36900 per QALY and $41800 per QALY for women with predicted 10-year survival between 75% and 99% (corresponding to women aged 70–74 years with no comorbidity). The ICER for EBRT increased to greater than $63800 per QALY for women with a predicted 10-year survival of less than 25%.
Figure 2.
Cost-effectiveness of external beam radiation therapy (EBRT) and newer modalities based on patient age and comorbidity. Solid bars represent the ranges of incremental cost-effectiveness ratio for EBRT, and dotted bars represent those for intensity modulated radiation therapy (IMRT), according to patient age and comorbidity. Conditions used to create comorbidity categories included congestive heart failure, cardiac arrhythmias, valvular disease, pulmonary circulation disorders, peripheral vascular disorders, paralysis, other neurological disorders, chronic pulmonary disease, diabetes, renal failure, liver disease, AIDS/human immunodeficiency virus, lymphoma, metastatic cancer, solid tumor without metastasis, rheumatoid arthritis/collagen, coagulopathy, weight loss, fluid and electrolyte disorders, deficiency anemia, alcohol abuse, drug abuse, psychoses, depression. We calculated the actual survival for each age group and comorbidity combination. Each combination was categorized into four groups based on 10-year survival (0%–25%, 25%–50%, 50%–75%, 75%–100%). A series of simulations integrating the corresponding mortality rate and age estimated the cost-effectiveness of EBRT (or IMRT) across these four categories. We assumed IMRT did not reduce the risk of local recurrence (compared with EBRT) but increased utility by 25% for the health state of no recurrence. QALY = quality-adjusted life-year.
The mean cancer-related costs per patient receiving newer RT modalities were $23605 for IMRT and $23628 for brachytherapy. Because the costs were similar, we only compared IMRT with EBRT. Assuming IMRT was able to completely prevent a recurrence (ie, recurrence probability is zero) but did not change the utilities of the health states, IMRT remained less cost-effective, with ICERs greater than $100000 per QALY for women aged greater than 70 years. Assuming IMRT was identical to EBRT in preventing recurrence and IMRT increased the utility of receiving RT in a no recurrence state by 25% (compared with healthy women without breast cancer), the ICERs of IMRT (compared with EBRT) were $77100 per QALY for women aged 70 years, $86700 per QALY for women aged 75 years, and $110500 per QALY for women aged 80 years (Table 2).
The ICER of the newer modalities also increased with decreasing 10-year survival probability (Figure 2). Specifically, the cost-effectiveness estimates for IMRT were between $70200 per QALY and $79300 per QALY for women aged 70 years with a predicted survival between 75% and 99%, assuming IMRT increases the utility of baseline state by 25%. Under the same assumption, in women with a predicted survival less than 25%, the cost-effectiveness estimates for IMRT were greater than $119700 per QALY (Figure 2).
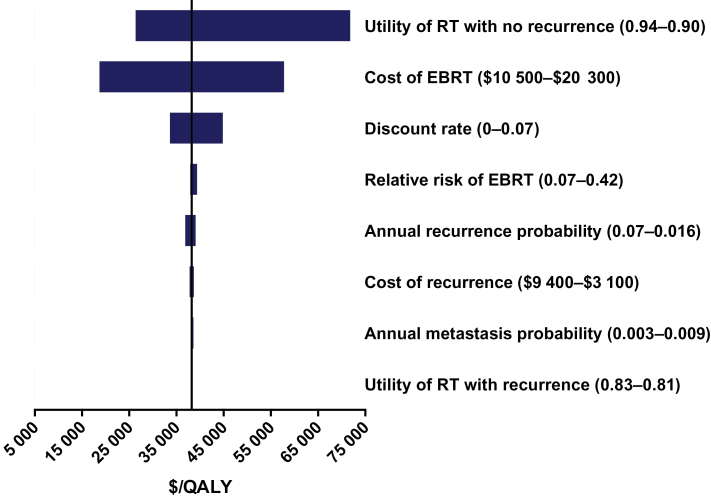
The cost-effectiveness estimates were sensitive to the utility of RT in women who had no recurrence. That is, when the incremental utility of RT was decreased by 50% in women with no recurrence, the ICER for EBRT increased to $77200 per QALY (Figure 3). Our estimates were also sensitive to the cost of EBRT. When the recurrence probabilities were derived from the SEER–Medicare sample, the ICER for EBRT increased by 6% to $43100 per QALY for women aged 70 years. Variations in the other variables such as survival changed our estimates by less than 5%.
Figure 3.
One-way sensitivity analysis for external beam radiation therapy (EBRT) in women aged 70 to 74 years. Bars indicate range of costs per quality-adjusted life-year (QALY) for a given range-specific model input variables. RT = radiation therapy.
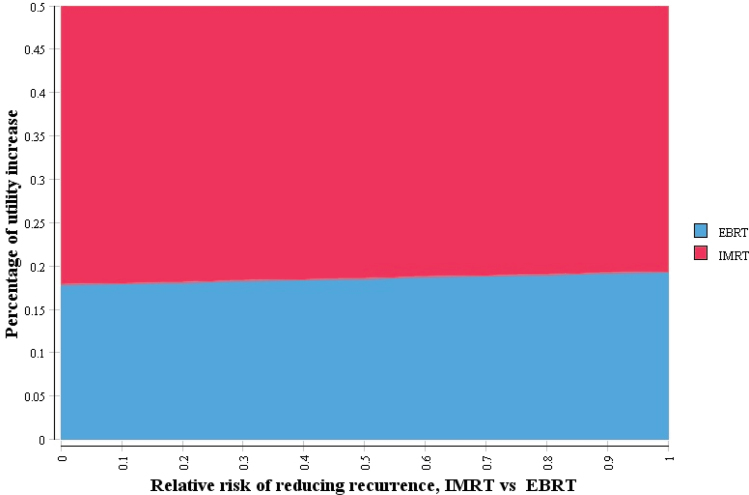
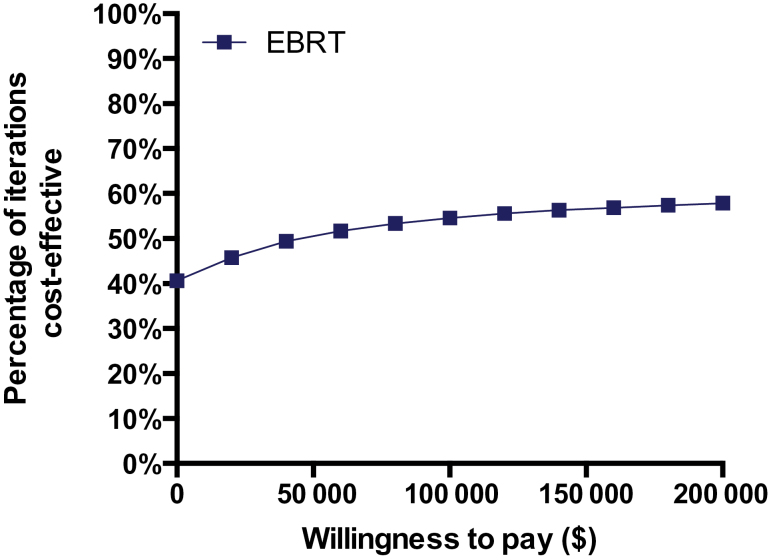
The two-way sensitivity analysis showed that reduction in recurrence played a minor, if any, role in terms of IMRT cost-effectiveness (Figure 4). IMRT would be cost-effective when it could increase QoL by approximately 20% of the utility difference between women without breast cancer and women with breast cancer receiving RT with no recurrence. Probabilistic sensitivity analysis demonstrated that EBRT had a 54.6% probability of cost-effectiveness over no RT at a willingness-to-pay threshold of $100000 per QALY for women aged 70 years (Figure 5).
Figure 4.
Two-way sensitivity analysis of net benefits using willing-to-pay (WTP) of $100000 per quality-adjusted life-year (QALY) between percentage of utility increase and relative risk of intensity modulated radiation therapy (IMRT) in reducing recurrence. The upper area indicates combinations where IMRT is cost-effective with a WTP threshold of $100000 per QALY, and lower area indicates combinations where external beam radiation therapy (EBRT) is cost-effective with the same WTP threshold constraint.
Figure 5.
Cost-effectiveness acceptability curve is shown from probabilistic sensitivity analysis of external beam radiation therapy (EBRT), women aged 70 to 74 years. The squares indicate the percentage of iterations out of 100 000 iterations that were cost-effective at a given willingness to pay threshold during a probabilistic sensitivity analysis.
Discussion
Despite concerns about “overuse” of RT in the older population, we found that EBRT is generally a cost-effective therapy for older women with early-stage breast cancer. The ICER of $44600 per QALY for EBRT falls below the willingness-to-pay benchmark of $50000 per QALY that is typically considered acceptable (23,35). However, we found substantial variability in the cost-effectiveness of EBRT when considering variation in expected survival based on age and comorbidity, with ICERs surpassing both $50000 per QALY and a more conservative benchmark of $100000 per QALY with increasing age and comorbidity. Although there is substantial interest in identifying both cancer characteristics and treatment factors that are predictive of recurrence, our results highlight that expected survival is of critical importance in assessing treatment value. Reliable tools to assess survival are needed to facilitate decision-making for older women.
Our results also raise important questions about the cost-effectiveness of IMRT and brachytherapy. Noting the absence of effectiveness data, our model estimated that the newer modalities would have to improve QALYs gained by at least 37% to be cost-effective. At present, however, there is no evidence that these newer modalities are 37% more effective in reducing recurrence risk or improving survival or QoL. Importantly, our study found that the utility benefit of RT over no RT was an important driver for cost effectiveness. We further found that reducing recurrence did not reduce the ICER substantially given the low local recurrence rate in this population. Instead, IMRT would have to improve the utility of receiving RT in a no-recurrence state by at least 20% to be cost-effective (<$100000 per QALY). This study demonstrates a unique approach to evaluating newer cancer treatments as they diffuse into clinical practice with limited effectiveness data. By using Medicare and trial data to determine total cancer-related costs and clinical effectiveness parameters, this approach can be more broadly applied to other novel cancer treatments to inform patients, practitioners, and policy makers on cost-effective care and effectiveness goals of ongoing clinical trials.
There are important limitations to consider. Our assumptions regarding utilities do not take into consideration different recurrence risks with current therapies, differential complication profiles of newer therapies, and how patient preferences change with age, factors that can all affect utilities (36,37). The utilities we used were from a study in which patients reported a QoL benefit with RT, presumably from the peace of mind that RT allowed. Although RT does lower the recurrence risk in older women, it remains unclear how much RT improves QoL for women for whom there may be no survival benefit. Considering a patient-specific risk tolerance, older women who are very risk averse may be unlikely to accept a higher recurrence risk by omitting RT and thereby have a long-term QoL benefit by receiving RT. Our analyses highlight the importance of understanding QoL effects after RT because the ICERs were very sensitive to patients’ utilities after RT. These estimates would be strengthened with more information on how utilities are affected by age and receipt of newer RT modalities along with long-term data regarding the efficacy of these newer modalities.
Clinical and patient-reported outcomes data are also needed to understand short-term and long-term factors that affect the cost-effectiveness of various RT modalities. If patients place great importance on avoiding short-term toxicity or upon shorter RT schedules (such as with brachytherapy), they may feel that more expensive treatment is justified, despite a lack of a longer-term benefit. For example, if brachytherapy is associated with less severe acute skin reactions compared with EBRT, this may lead to a transiently increased utility for brachytherapy but a minimal effect on the long-term QALY forecast. In contrast, if brachytherapy substantially improves long-term breast outcomes such as cosmesis or late effects on the lung and heart, the effect on QALYs could be more substantial. Other factors that may affect long-term utility and costs of newer modalities include subsequent related procedures, screening, and late toxicity (22,38,39). Thus, there is a need to assess patient-reported outcomes to improve our understanding of the utility of newer modalities.
We determined costs from a payer perspective because of the substantial interest in Medicare expenditures for cancer care and concerns about overall trends in Medicare costs. However, beneficiaries are also subject to opportunity costs such as time and costs from lost wages that our analysis does not capture. Future research should incorporate information regarding these costs. Finally, our analysis was confined to a 10-year time horizon because the trial-based effectiveness measures we used have only been reported for this time period. Future work incorporating longer-term effectiveness data that enable simulations over a lifetime horizon will strengthen cost-effectiveness estimates.
In summary, EBRT is generally cost-effective for older women with early-stage breast cancer, but notably less cost-effective for women with a high comorbidity burden and diminished life expectancy. Newer modalities such as IMRT and brachytherapy are costlier than EBRT and would have to be substantially more effective in improving cancer control or QoL to be cost-effective. As newer technologies disseminate into clinical practice, it will be important to provide data on comparative effectiveness relative to costs to better inform clinical decision-making.
Funding
This study was supported by the National Cancer Institute (R01CA149045) and the P30 Cancer Center Support Grant (CCSG) at the Yale Comprehensive Cancer Center (P30CA016359). The collection of the California cancer incidence data used in this study was supported by the California Department of Public Health as part of the statewide cancer reporting program mandated by California Health and Safety Code Section 103885; the National Cancer Institute’s Surveillance, Epidemiology and End Results Program under contract N01-PC-35136 awarded to the Northern California Cancer Center, contract N01-PC-35139 awarded to the University of Southern California, and contract N02-PC-15105 awarded to the Public Health Institute; and the Centers for Disease Control and Prevention’s National Program of Cancer Registries, under agreement U55/CCR921930-02 awarded to the Public Health Institute.
The ideas and opinions expressed herein are those of the author(s) and endorsement by the State of California, Department of Public Health the National Cancer Institute, and the Centers for Disease Control and Prevention or their contractors and subcontractors is not intended nor should be inferred.
The study sponsor (NIH) did not play a role in the design of the study; the collection, analysis, or interpretation of the data; the writing of the manuscript; or the decision to submit the manuscript for publication. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
We acknowledge the efforts of the Applied Research Program, National Cancer Institute; the Office of Research, Development and Information, Centers for Medicare and Medicaid Services; Information Management Services, Inc.; and the SEER Program tumor registries in the creation of the SEER–Medicare database. The interpretation and reporting of the SEER–Medicare data are the sole responsibility of the authors.
References
- 1. Clarke M, Collins R, Darby S, et al. Effects of radiotherapy and of differences in the extent of surgery for early breast cancer on local recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005;366(9503):2087–2106 [DOI] [PubMed] [Google Scholar]
- 2. Hughes KS, Schnaper LA, Berry D, et al. Lumpectomy plus tamoxifen with or without irradiation in women 70 years of age or older with early breast cancer. N Engl J Med. 2004;351(10):971–977 [DOI] [PubMed] [Google Scholar]
- 3. Hughes KS, Schnaper LA, Cirrincione C, et al. Lumpectomy plus tamoxifen with or without irradiation in women age 70 or older with early breast cancer. J Clin Oncol. 2010;28(Suppl.15s):abstract 507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Fisher B, Jeong JH, Anderson S, et al. Twenty-five-year follow-up of a randomized trial comparing radical mastectomy, total mastectomy, and total mastectomy followed by irradiation. N Engl J Med. 2002;347(8):567–575 [DOI] [PubMed] [Google Scholar]
- 5. Njeh CF, Saunders MW, Langton CM. Accelerated partial breast irradiation (APBI): a review of available techniques. Radiat Oncol. 2010;5:90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Aristei C, Palumbo I, Cucciarelli F, et al. Partial breast irradiation with interstitial high-dose-rate brachytherapy in early breast cancer: results of a phase II prospective study. Eur J Surg Oncol. 2009;35(2):144–150 [DOI] [PubMed] [Google Scholar]
- 7. Bartelink H, Horiot JC, Poortmans P, et al. Recurrence rates after treatment of breast cancer with standard radiotherapy with or without additional radiation. N Engl J Med. 2001;345(19):1378–1387 [DOI] [PubMed] [Google Scholar]
- 8. Abbott AM, Habermann EB, Tuttle TM. Trends in the use of implantable accelerated partial breast irradiation therapy for early stage breast cancer in the United States. Cancer. 2011;117(15):3305–3310 [DOI] [PubMed] [Google Scholar]
- 9. Carlson RW, McCormick B. Update: NCCN breast cancer clinical practice guidelines. J Natl Compr Canc Netw. 2005;3(Suppl 1):S7–S11 [PubMed] [Google Scholar]
- 10. Carlson RW, Allred DC, Anderson BO, et al. Invasive breast cancer. J Natl Compr Canc Netw. 2011;9(2):136–222 [DOI] [PubMed] [Google Scholar]
- 11. Hughes KS, Schnaper LA, Bellon JR, et al. Lumpectomy plus tamoxifen with or without irradiation in women age 70 years or older with early breast cancer: long-term follow-up of CALGB 9343. J Clin Oncol. 2013;31(19):2382–2387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Soulos PR, Yu JB, Roberts KB, et al. Assessing the impact of a cooperative group trial on breast cancer care in the medicare population. J Clin Oncol. 2012; 30(14):1601–1607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Roberts KB, Soulos PR, Herrin J, et al. The adoption of new adjuvant radiation therapy modalities among medicare beneficiaries with breast cancer: clinical correlates and cost implications. Int J Radiat Oncol Biol Phys. 2012; 85(5):1186–1192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Suh WW, Pierce LJ, Vicini FA, et al. A cost comparison analysis of partial versus whole-breast irradiation after breast-conserving surgery for early-stage breast cancer. Int J Radiat Oncol Biol Phys. 2005;62(3):790–796 [DOI] [PubMed] [Google Scholar]
- 15. Hattangadi JA, Taback N, Neville BA, et al. Accelerated partial breast irradiation using brachytherapy for breast cancer: patterns in utilization and guideline concordance. J Natl Cancer Inst. 2012;104(1):29–41 [DOI] [PubMed] [Google Scholar]
- 16. Smith GL, Xu Y, Buchholz TA, et al. Brachytherapy for accelerated partial-breast irradiation: a rapidly emerging technology in breast cancer care. J Clin Oncol. 2011;29(2):157–165 [DOI] [PubMed] [Google Scholar]
- 17. Beitsch PD, Shaitelman SF, Vicini FA. Accelerated partial breast irradiation. J Surg Oncol. 2011;103(4):362–368 [DOI] [PubMed] [Google Scholar]
- 18. Shaitelman SF. Sounding a warning bell? Documentation of the increased utilization of accelerated partial breast irradiation. J Natl Cancer Inst. 2012;104(1):5–7 [DOI] [PubMed] [Google Scholar]
- 19. Smith GL, Xu Y, Buchholz TA, et al. Association between treatment with brachytherapy vs whole-breast irradiation and subsequent mastectomy, complications, and survival among older women with invasive breast cancer. JAMA. 2012;307(17):1827–1837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Presley CJ, Soulos PR, Herrin J, et al. Patterns of use and short-term complications of breast brachytherapy in the national Medicare population from 2008–2009. J Clin Oncol. 2012; 30(35):4302–4307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Donovan E, Bleakley N, Denholm E, et al. Randomised trial of standard 2D radiotherapy (RT) versus intensity modulated radiotherapy (IMRT) in patients prescribed breast radiotherapy. Radiother Oncol. 2007;82(3):254–264 [DOI] [PubMed] [Google Scholar]
- 22. Barnett GC, Wilkinson JS, Moody AM, et al. Randomized controlled trial of forward-planned intensity modulated radiotherapy for early breast cancer: interim results at 2 years. Int J Radiat Oncol Biol Phys. 2012;82(2):715–723 [DOI] [PubMed] [Google Scholar]
- 23. Grosse SD. Assessing cost-effectiveness in healthcare: history of the $50,000 per QALY threshold. Expert Rev Pharmacoecon Outcomes Res. 2008;8(2):165–178 [DOI] [PubMed] [Google Scholar]
- 24. Neumann PJ. What next for QALYs? JAMA. 2011;305(17):1806–1807 [DOI] [PubMed] [Google Scholar]
- 25. Neumann PJ. Using Cost-Effectiveness Analysis to Improve Health Care: Opportunities and Barriers. New York: Oxford University Press; 2005 [Google Scholar]
- 26. Warren JL, Klabunde CN, Schrag D, et al. Overview of the SEER–Medicare data: content, research applications, and generalizability to the United States elderly population. Med Care. 2002;40(8 Suppl):IV–3–18. [DOI] [PubMed] [Google Scholar]
- 27. Rao S, Kubisiak J, Gilden D. Cost of illness associated with metastatic breast cancer. Breast Cancer Res Treat. 2004;83(1):25–32 [DOI] [PubMed] [Google Scholar]
- 28. Warren JL, Brown ML, Fay MP, et al. Costs of treatment for elderly women with early-stage breast cancer in fee-for-service settings. J Clin Oncol. 2002;20(1):307–316 [DOI] [PubMed] [Google Scholar]
- 29. Yabroff KR, Lamont EB, Mariotto A, et al. Cost of care for elderly cancer patients in the United States. J Natl Cancer Inst. 2008;100(9):630–641 [DOI] [PubMed] [Google Scholar]
- 30. Hayman JA, Fairclough DL, Harris JR, et al. Patient preferences concerning the trade-off between the risks and benefits of routine radiation therapy after conservative surgery for early-stage breast cancer. J Clin Oncol. 1997;15(3):1252–1260 [DOI] [PubMed] [Google Scholar]
- 31. Folland S, Goodman AC, Stano M. The Economics of Health and Health Care. 5th ed. Upper Saddle River, NJ: Pearson Prentice Hall; 2007 [Google Scholar]
- 32. Stout NK, Rosenberg MA, Trentham-Dietz A, et al. Retrospective cost-effectiveness analysis of screening mammography. J Natl Cancer Inst. 2006;98(11):774–782 [DOI] [PubMed] [Google Scholar]
- 33. Elixhauser A, Steiner C, Harris DR, et al. Comorbidity measures for use with administrative data. Med Care. 1998;36(1):8–27 [DOI] [PubMed] [Google Scholar]
- 34. Briggs AH, Goeree R, Blackhouse G, et al. Probabilistic analysis of cost-effectiveness models: choosing between treatment strategies for gastroesophageal reflux disease. Med Decis Making. 2002;22(4):290–308 [DOI] [PubMed] [Google Scholar]
- 35. Jena AB, Philipson TJ. Cost-effectiveness analysis and innovation. J Health Econ. 2008;27(5):1224–1236 [DOI] [PubMed] [Google Scholar]
- 36. Cohen HJ, Lan L, Archer L, et al. Impact of age, comorbidity and symptoms on physical function in long-term breast cancer survivors (CALGB 70803). J Geriatr Oncol. 2012;3(2):82–89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Mohile SG, Heckler C, Fan L, et al. Age-related differences in symptoms and their interference with quality of life in 903 cancer patients undergoing radiation therapy. J Geriatr Oncol. 2011;2(4):225–232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Evans SB, Kaufman SA, Price LL, et al. Persistent seroma after intraoperative placement of MammoSite for accelerated partial breast irradiation: incidence, pathologic anatomy, and contributing factors. Int J Radiat Oncol Biol Phys. 2006;65(2):333–339 [DOI] [PubMed] [Google Scholar]
- 39. Ravi A, Lee S, Karsif K, et al. Intraoperative placement of MammoSite for breast brachytherapy treatment and seroma incidence. Brachytherapy. 2010;9(1):76–80 [DOI] [PubMed] [Google Scholar]
- 40. SEER Cancer Statistics Review http://seer.cancer.gov/csr/1975_2005/results_merged/sect_04_breast.pdf Accessed January 10, 2013.