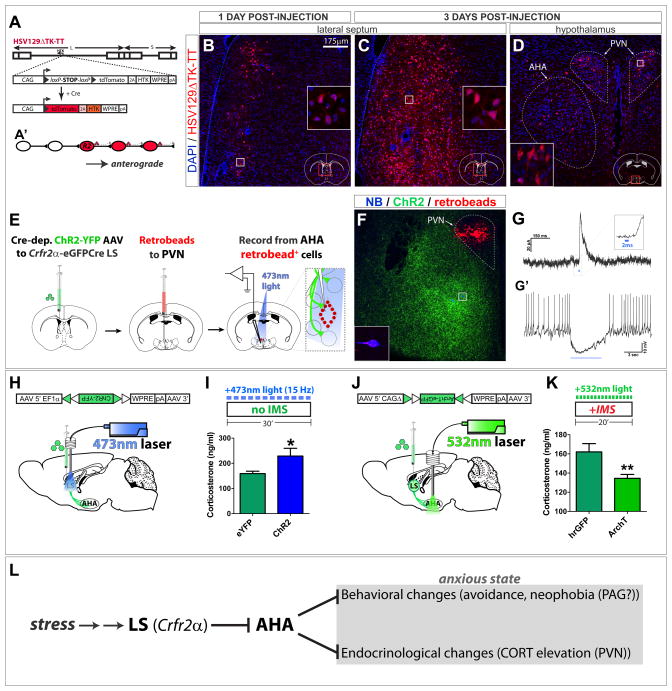
Figure 7. LS Crfr2α+ projections to AHA are polysynaptically upstream of PVN and positively regulate corticosterone levels.
(A,A′) HSV129ΔTK-TT.
(B–D) tdT (red) in HSV129ΔTK-TT-injected Crfr2α-eGFPCre mice counterstained with DAPI (blue) at 1 (B) or 3 (C,D) days post-injection (dpi) in LS (B,C) or medial hypothalamus (D). Dashed lines in (D) demarcate PVN (upper right) and AHA (lower left).
(E–G) Combined CRACM and retrograde tracing. (F) A slice used for recording; Crfr2α+ChR2-YFP+ axons originating from LS (green), retrobeads (red), and two neurobiotin-filled neurons (blue). The cell that yielded the traces in (G) is shown at higher magnification in the inset. (G) A 2ms pulse of 473nm light induced monosynaptic IPSCs in 4/9 retrobead+ AHA neurons that were sufficient to inhibit firing (G′).
(H,I) Optogenetic stimulation of LS Crfr2α+ neurons increased CORT. eYFP(n=9) vs. ChR2(n=9): CORT, ng/ml (159.2±9.3 vs. 228.8±30.9, P<0.05).
(J,K) Optogenetic inhibition of LS Crfr2α+ projections to AHA decreased CORT. hrGFP(n=15) vs. ArchT-GFP(n=17): CORT, ng/ml (162.1±8.6 vs. 134.5±4.1, P<0.01).
Values indicate mean±s.e.m.
(L) Proposed model for LS Crfr2α+ neuronal control of stress-induced anxiety.
See also Figure S7.