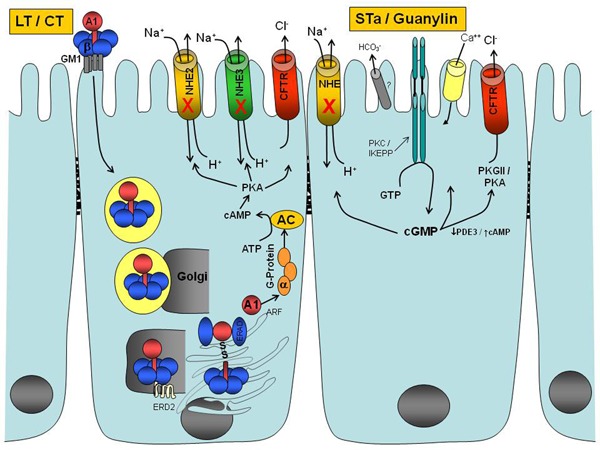
Figure 1. Mechanisms of action of the cholera toxin (CT), heat-labile (LT) and heat-stable enterotoxin (STa) from Escherichia coli, and endogenous peptide ligands of guanylyl cyclase C (GC-C). CT or LT binds to the monosialoganglioside GM1 receptor at the host mucosa surface and triggers endocytosis of the holotoxin. The A1 domain of the A subunit is transported through the Golgi complex and endoplasmic reticulum to activate the Gsα subunit of G-protein. This A1 domain interacts with ADP-ribosylating factors to ADP-ribosylate this Gsα subunit in order to activate G-protein and consequently adenylyl cyclase (AC). The AC cleaves ATP to cAMP and subsequently activates protein kinase A, which inhibits NaCl absorption (NHE transporters) and increases chloride secretion through the cystic fibrosis transmembrane regulator (CFTR). Peptide ligands of the extracellular domain of GC-C activate the intracellular catalytic domain of GC-C resulting in cGMP formation, which activates several pathways: a) inhibition of the NHE3 transporter, which decreases NaCl absorption; b) activation of CFTR, which leads to secretion of chloride; and c) increased calcium influx.