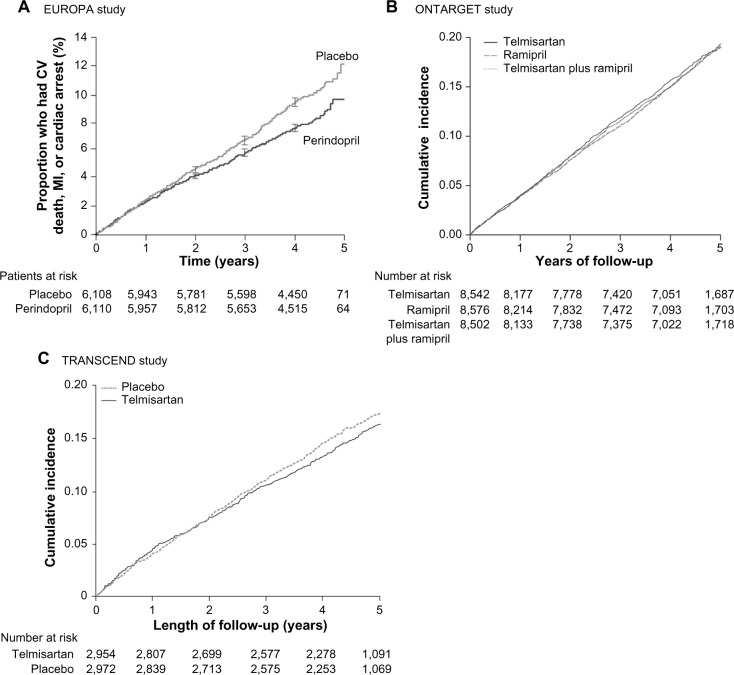
Figure 2.
Primary outcome* results in the EUROPA,31 ONTARGET,35 and TRANSCEND33 studies. (A) The EUROPA study assessed superiority of perindopril versus placebo. (B) The ONTARGET study assessed noninferiority of telmisartan versus ramipril defined as an HR below the predefined margin. (C) The TRANSCEND study assessed whether telmisartan compared to placebo reduces the primary outcome occurrence in patients with CV disease or high-risk diabetes and without HF who are intolerant to ACE inhibitors.
Notes: *CV death, MI, or cardiac arrest in EUROPA; composite outcome of death from CV causes, MI, stroke, or hospitalization for HF in ONTARGET and TRANSCEND. (A) Reprinted from The Lancet, 362, Fox KM, Efficacy of perindopril in reduction of cardiovascular events among patients with stable coronary artery disease: randomised, doubleblind, placebo-controlled, multicentre trial (the EUROPA study), 782–788, copyright © (2003), with permission from Elsevier.31 (B) Reprinted from rom N Engl J Med, Yusuf S, Teo KK, Pogue J, et al, Telmisartan, ramipril, or both in patients at high risk for vascular events, 358, 1547–1559, copyright © (2008) Massachusetts Medical Society. Reprinted with permission from Massachusetts Medical Society.35 (C) Reprinted from The Lancet, 372, Yusuf S, Teo K, Anderson C, et al, Effects of the angiotensin-receptor blocker telmisartan on cardiovascular events in high-risk patients intolerant to angiotensin-converting enzyme inhibitors: a randomised controlled trial, 1174–1183, copyright (2008), with permission from Elsevier.33
Abbreviations: ACE, angiotensin-converting enzyme; CV, cardiovascular; EUROPA, EURopean trial On reduction of cardiac events with Perindopril in stable coronary Artery disease; HF, heart failure; HR, hazard ratio; MI, myocardial infarction; ONTARGET, ONgoing Telmisartan Alone and in combination with Ramipril Global Endpoint Trial; TRANSCEND, Telmisartan Randomized AssessmeNt Study in ACE-I iNtolerant subjects with cardiovascular Disease.