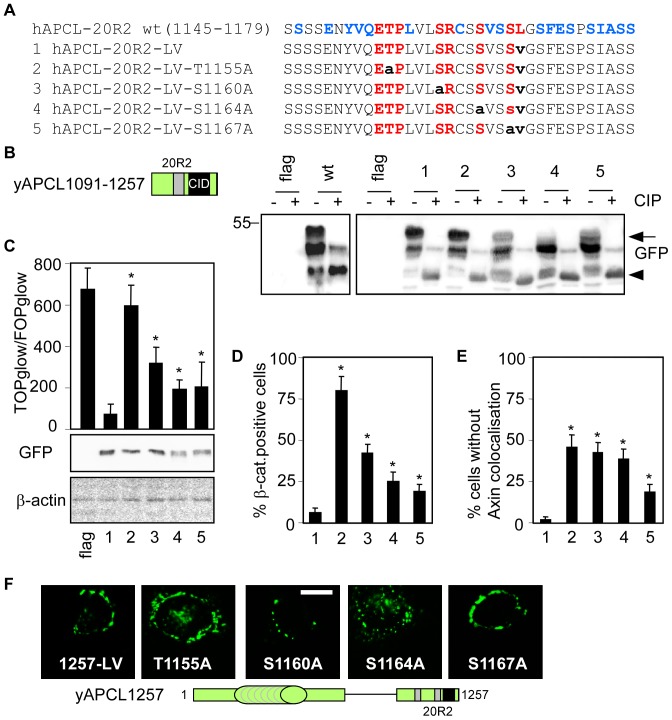
Figure 6. The residues of the 20R2 required for APCL phosphorylation are also required to inhibit β-catenin transcriptional activity, to target β-catenin for degradation and to interact with Axin.
A, The sequences of the wild-type and mutant 20R2 from APCL (residues 1145–1179). The constructs are numbered in B–E. Bold capital letters indicate residues common to APC and APCL from various species (fig. S2), and small bold letters signify residues that have been mutated. B, Amino acids within the 20R2 that were involved in the phosphorylation of truncated APCL. 293T cells were transiently transfected on day 1 with the indicated yAPCL(1091–1257) constructs. On day 3, cell extracts were subjected to immunoprecipitation using an anti-GFP antibody followed by alkaline phosphatase treatment (CiP) and western blotting using the anti-GFP antibody. The arrow indicates the phosphorylated APCL fragments that were affected by mutation, and the arrowhead points to non-phosphorylated APCL constructs. C, SW480 cells were transiently transfected on day 1 with reporter plasmids and 100 ng of an empty vector (flag) or the indicated yAPCL1257 constructs. TOP/FOP reporter assays were performed on day 3 to measure β-catenin transcriptional activity. The data are presented as the mean ± standard deviation of three independent values from a representative experiment. * p<0.05 compared with mutant number 1, Student's t-test. In a parallel experiment, cells were transiently transfected with 1 μg of the indicated plasmids on day 1. Cell extracts were prepared on day 3 and were subjected to western blotting using the indicated antibodies. The molecular weights are shown in kDa. D, SW480 cells were transiently transfected with a control vector (flag) or the indicated yAPCL1257 constructs, fixed on day 3 and stained with an anti-β-catenin antibody. The percentages indicate the number of β-catenin-positive cells (n = 100), and the data are presented as the mean ± standard deviation of three independent experiments. * p<0.003 compared with mutant number 1, Student's t-test. E, SW480 cells were transiently transfected with the indicated yAPCL1257 constructs and N-terminal flag-tagged Axin on day 1. The cells were fixed on day 3 and were stained with an anti-flag antibody and Hoechst dye. The percentages indicate the number of cells without colocalisation (n = 100), and the data are presented as the mean ± standard deviation of three independent experiments. * p<0.009 compared with mutant number 1, Student's t-test. F, Intracellular localisation of the indicated yAPCL1257 constructs transfected as described in (D). Bar, 10 μM.