Figure 1. Chemical structure and stereo-configuration of the stereoisomers of diadenosine 5′,5″″-P1,P4-dithio-P2,P3-chloromethylenetetraphosphate (compound 1), and of adenosine 5′-(P1-thio-P2,P3-chloromethylenetriphosphate), (compound 2).
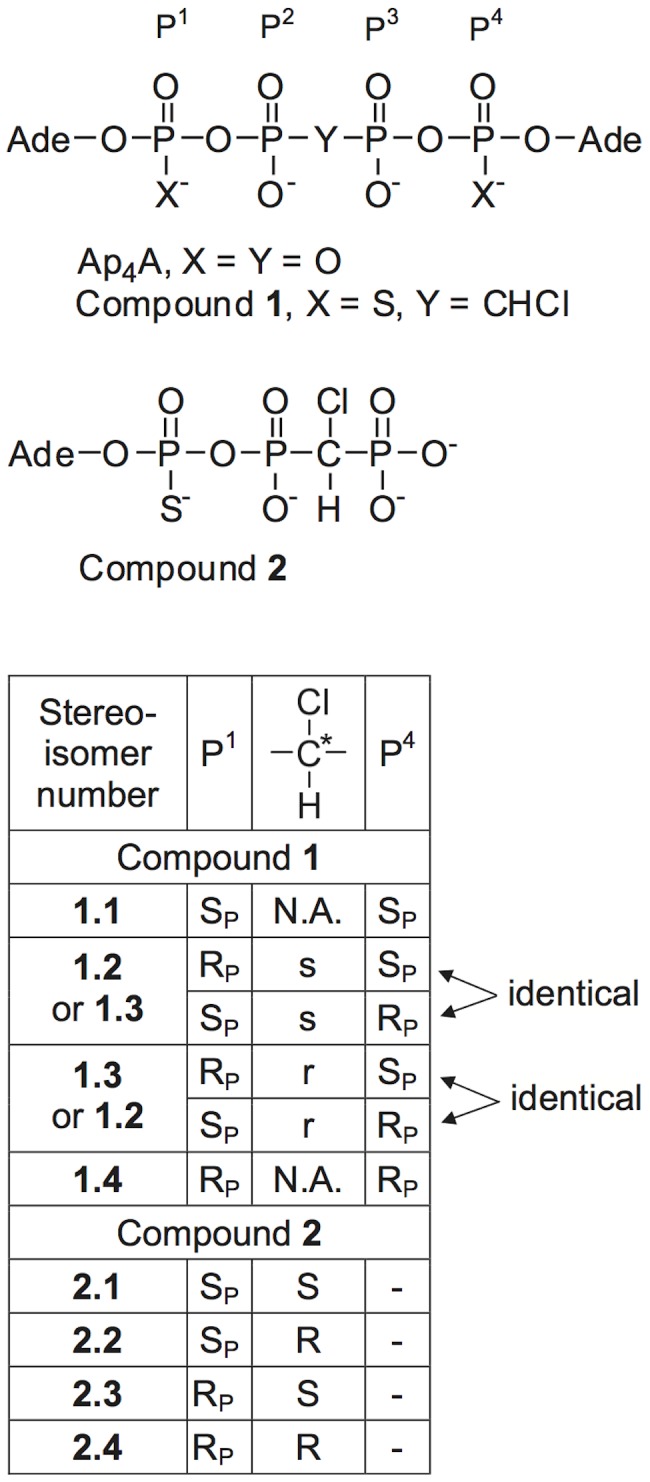
RP and SP designate the absolute configuration of chiral P1- and P4-phosphorothioates; r and s, the absolute configuration of the pseudo-asymmetric carbon of the P2,P3-chloromethylene group in compound 1; R and S, the absolute configuration of the chloromethylene group in compound 2. Ade, 5′-adenosyl; N.A., Not Asymmetric.
