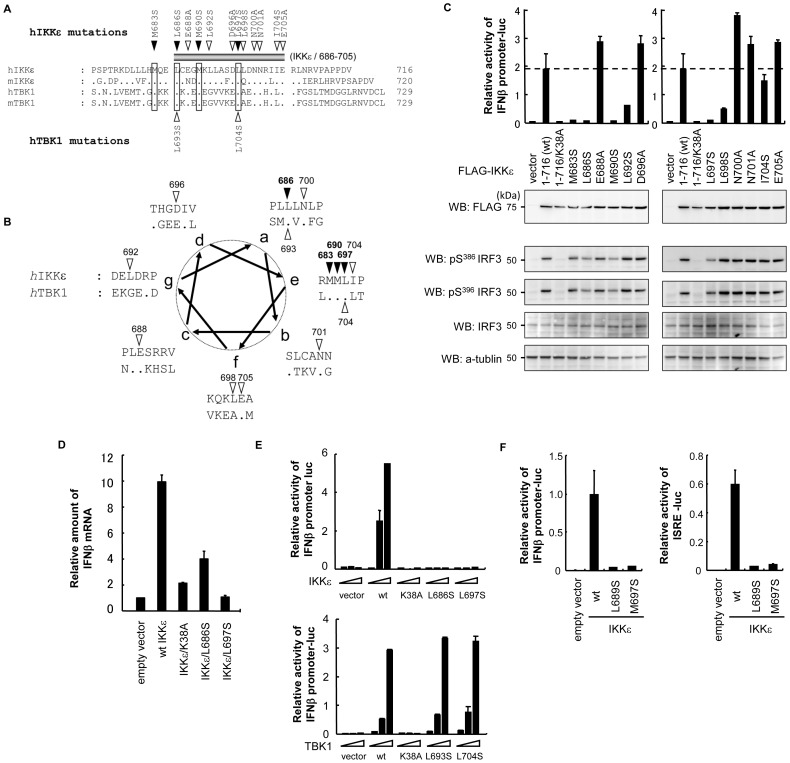
Figure 3. Mutation in the 686–705 region of IKKε, but not TBK1, had an effect on IFNβ promoter activity.
(A) Alignment of the C-terminal regions of human and murine IKK-related kinases. The putative functional domain in IKKε that is required for IFNβ promoter activity (686–705) is shown. The amino acid substitutions introduced into IKKε (top) or TKB1 (bottom) are also shown. (B) A helical model of the C-terminal regions of IKKε and TBK1. The mutated residues are indicated with triangles. (C) 293T cells were transfected with the IFNβ promoter-luciferase reporter along with FLAG-tagged wt IKKε, kinase defective mutant or mutants with the indicated single amino acid substitutions. Cells were lysed 24 h post-transfection and luciferase activities were quantified by normalization with renilla luciferase activity. The values represent the average of three samples +/− SD. Cell lysates were also subjected to SDS-PAGE and Western blot with the indicated antibodies. (D) L cells were transfected with indicated plasmid and total RNA were prepared at 24 h post-transfection. Relative amount of IFNβ mRNA were quantified by using qRT-PCR by normalization with HPRT mRNA. The values represent the average of three samples +/− SD. (E) 293T cells were transfected with the IFNβ promoter-luciferase reporter along with an increasing amount of the wt or mutant forms of TKB1 and IKKε. Luciferase activities were measured as shown in (C). (F) 293T cells were transfected with the IFNβ promoter-luciferase or ISRE-luciferase reporter along with the wt or mutant forms of IKKε. Luciferase activities were measured as shown in (C).