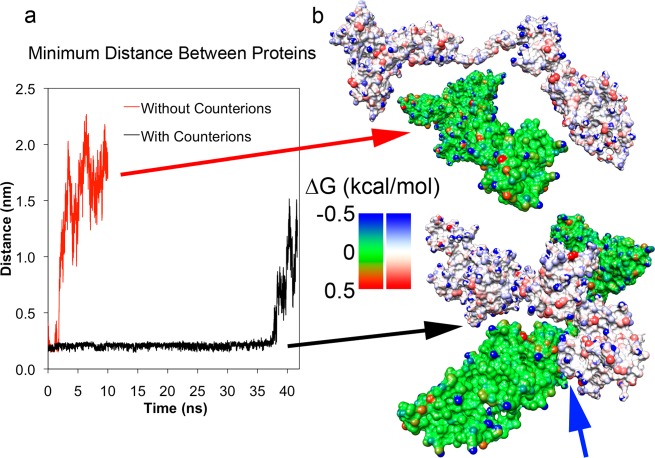
Figure 6.
Dimerization of two F conformation albumin proteins. (a) Minimum distance measured between two F-isoform BSA structures placed near each other and simulated with and without system neutralizing counterions. Proteins with counterions allowed proteins to stay within 0.25 nm of each other (black line) until they separated after 36 ns. Absence of counterions allowed unscreened repulsive electrostatic interactions to rapidly overcome attractions (red line). (b) Configurations of two proteins from (a) at 10 ns. The top pair (green protein and white protein) corresponds to the no counterion simulation and the bottom pair corresponds to the counterion simulation. Individual surface atoms are colored by the change in free energy due to solvation in water (kcal/mol). Hydrophobic and hydrophilic atoms are colored red and blue, respectively. The blue arrow indicates the point of contact between the two proteins.