Abstract
The Hsp70 family of molecular chaperones has an essential role in the synthesis, folding and translocation of the nascent peptide chain. While the general features of these activities are well documented, less is understood about the regulation of these activities. The ATPase rate is stimulated by non-native proteins, furthermore, interaction with ATP leads to the release of protein substrate concurrent with a conformational change in Hsp70. One interpretation of these data is that the two domains of Hsp70 interact. In the process of mapping the carboxyl-terminal boundary of the substrate binding domain for human Hsp70, we identified a regulatory motif, EEVD, which is conserved at the extreme carboxyl terminus among nearly all cloned cytosolic eukaryotic Hsp70s. Deletion or mutation of EEVD affects the ATPase activity, the ability to interact with substrates, and interferes with the ability of the mutant Hsp70 to interact with HDJ-1 in the refolding of denatured firefly luciferase. Examination of the biophysical properties of the mutant Hsp70s reveals a change in the overall shape and conformation of the protein consistent with reduced interactions between the two domains. These data suggest that the EEVD motif is involved in the intramolecular regulation of Hsp70 function and intermolecular interactions with HDJ-1.
Full text
PDF
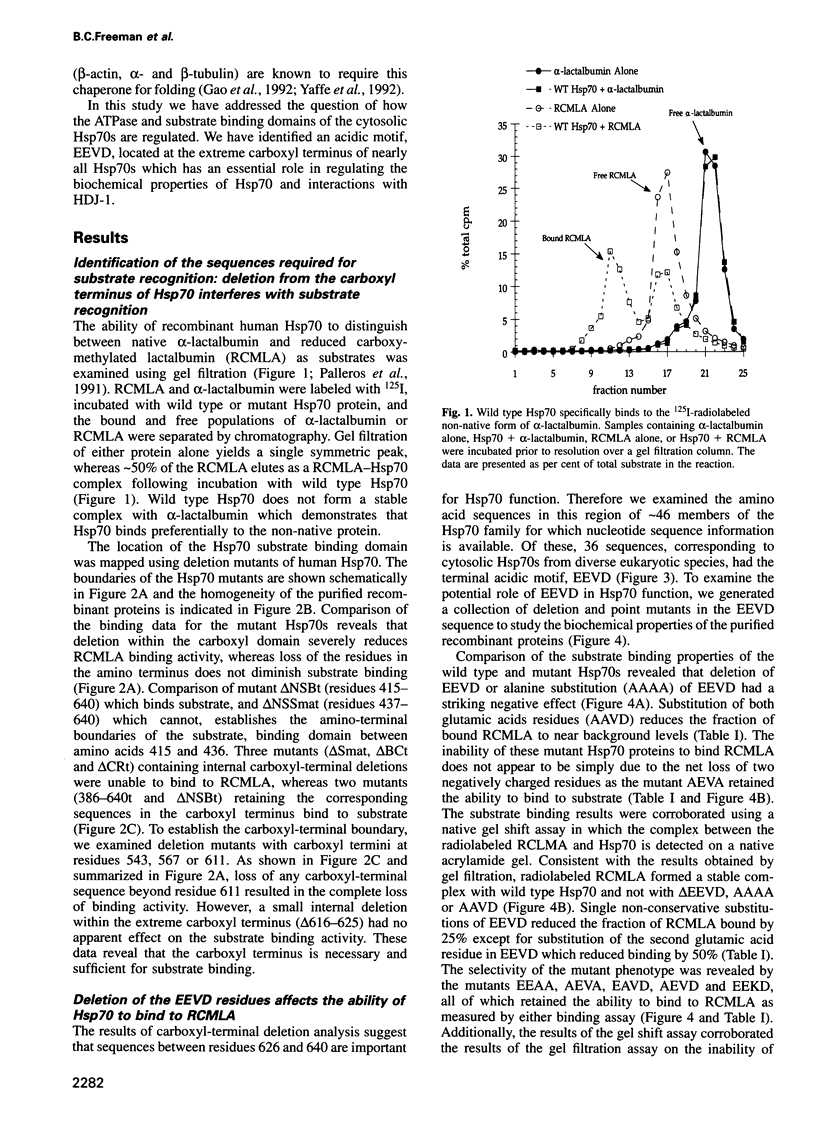
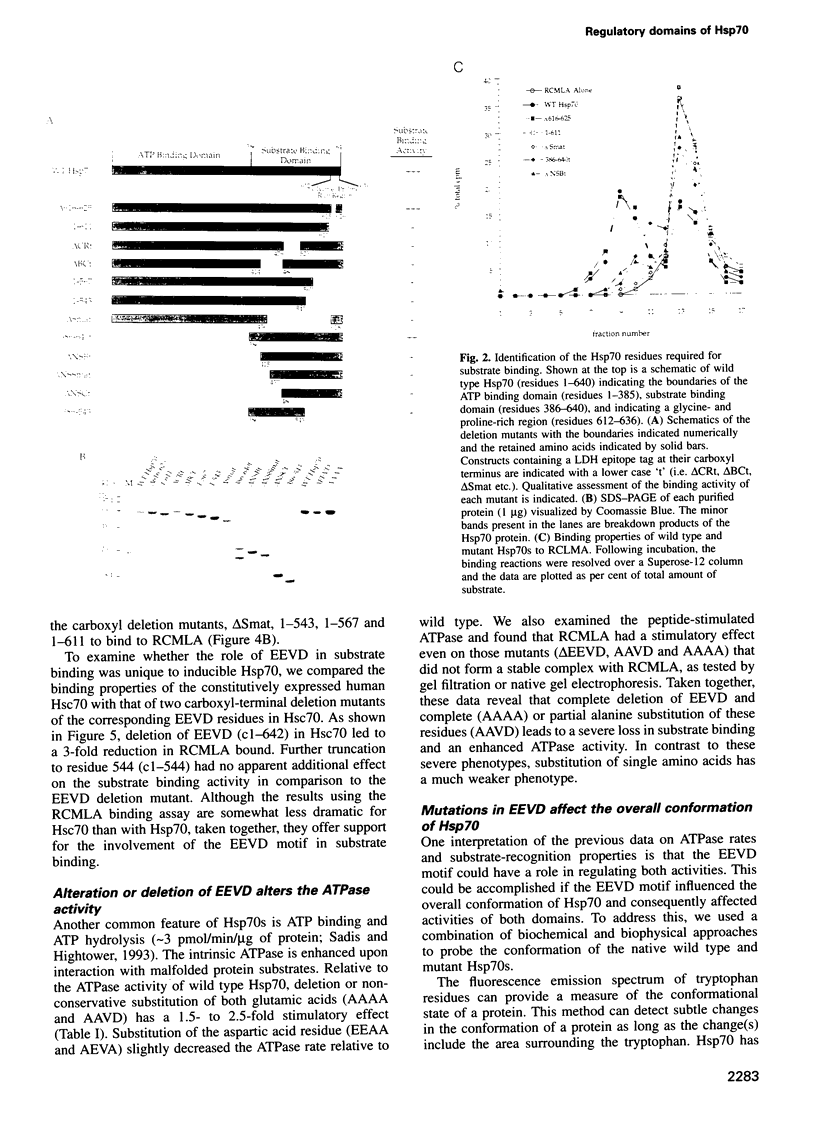

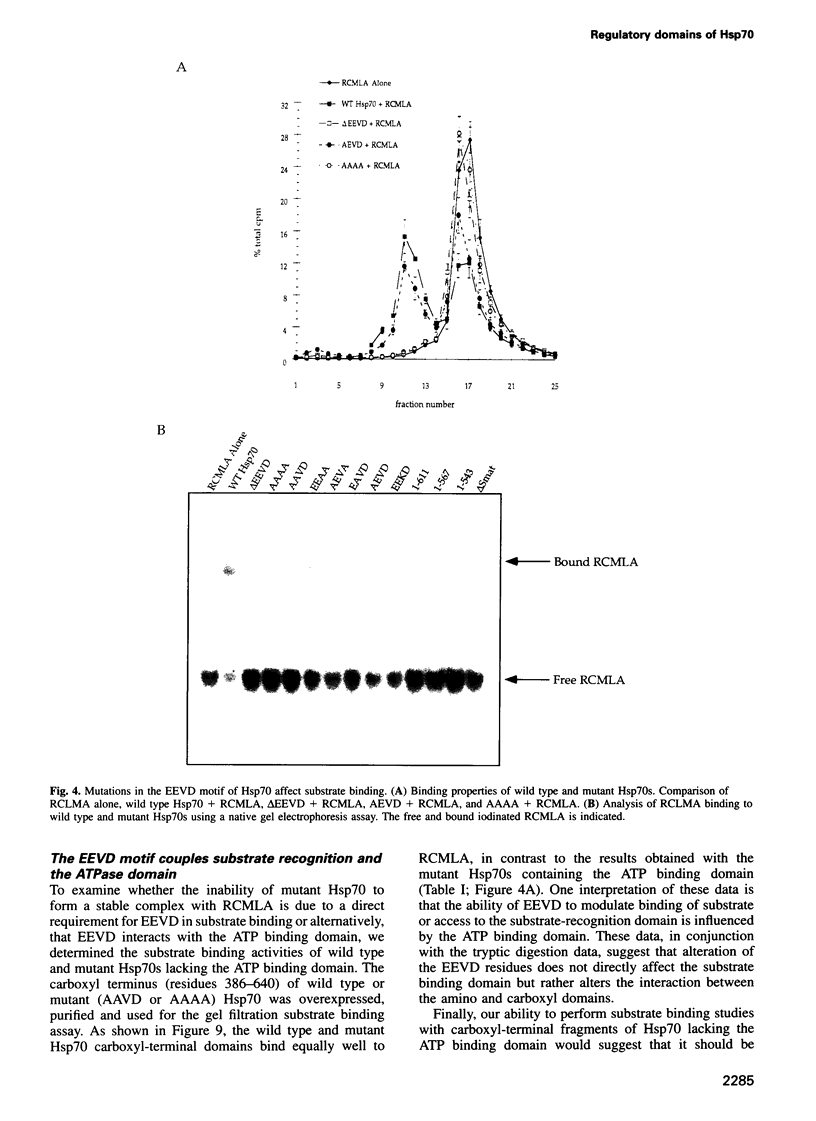
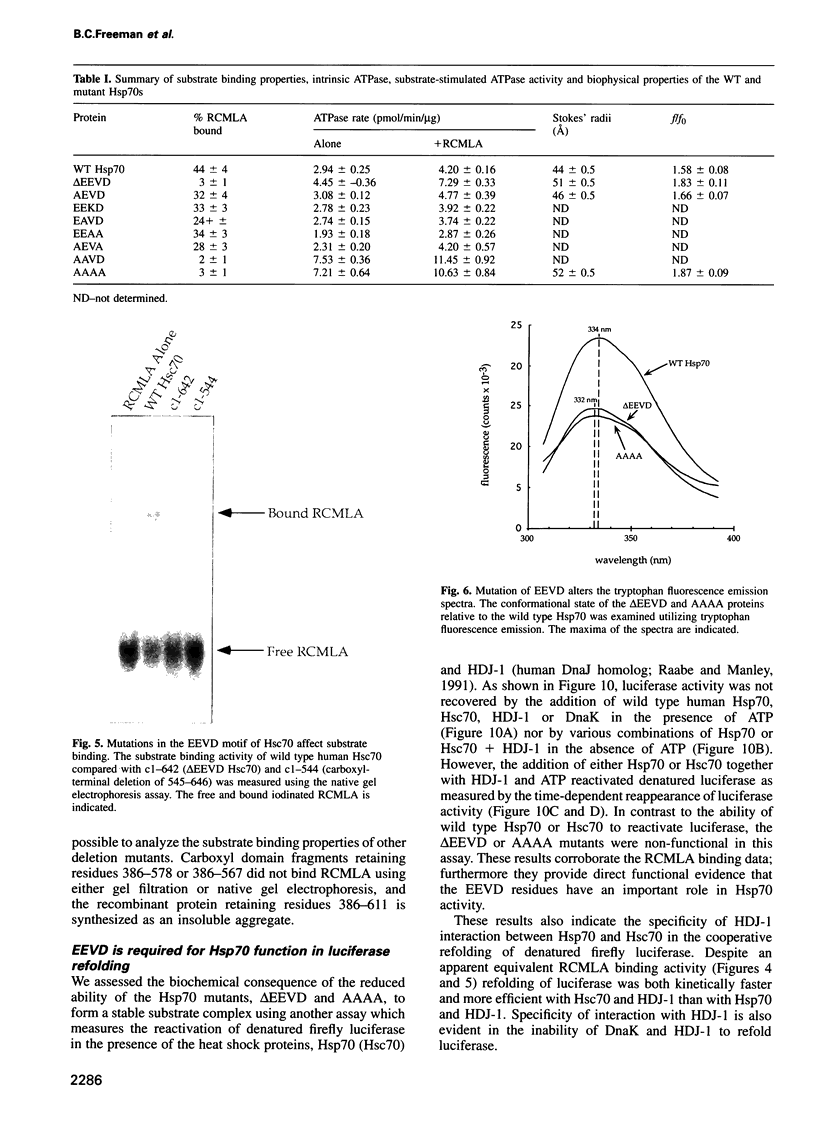





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abravaya K., Myers M. P., Murphy S. P., Morimoto R. I. The human heat shock protein hsp70 interacts with HSF, the transcription factor that regulates heat shock gene expression. Genes Dev. 1992 Jul;6(7):1153–1164. doi: 10.1101/gad.6.7.1153. [DOI] [PubMed] [Google Scholar]
- Beckmann R. P., Mizzen L. E., Welch W. J. Interaction of Hsp 70 with newly synthesized proteins: implications for protein folding and assembly. Science. 1990 May 18;248(4957):850–854. doi: 10.1126/science.2188360. [DOI] [PubMed] [Google Scholar]
- Bhattacharyya T., Karnezis A. N., Murphy S. P., Hoang T., Freeman B. C., Phillips B., Morimoto R. I. Cloning and subcellular localization of human mitochondrial hsp70. J Biol Chem. 1995 Jan 27;270(4):1705–1710. doi: 10.1074/jbc.270.4.1705. [DOI] [PubMed] [Google Scholar]
- Bienz M. Developmental control of the heat shock response in Xenopus. Proc Natl Acad Sci U S A. 1984 May;81(10):3138–3142. doi: 10.1073/pnas.81.10.3138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bresnick E. H., Sanchez E. R., Pratt W. B. Relationship between glucocorticoid receptor steroid-binding capacity and association of the Mr 90,000 heat shock protein with the unliganded receptor. J Steroid Biochem. 1988;30(1-6):267–269. doi: 10.1016/0022-4731(88)90104-5. [DOI] [PubMed] [Google Scholar]
- Buchberger A., Schröder H., Büttner M., Valencia A., Bukau B. A conserved loop in the ATPase domain of the DnaK chaperone is essential for stable binding of GrpE. Nat Struct Biol. 1994 Feb;1(2):95–101. doi: 10.1038/nsb0294-95. [DOI] [PubMed] [Google Scholar]
- Bukau B., Walker G. C. Mutations altering heat shock specific subunit of RNA polymerase suppress major cellular defects of E. coli mutants lacking the DnaK chaperone. EMBO J. 1990 Dec;9(12):4027–4036. doi: 10.1002/j.1460-2075.1990.tb07624.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caplan A. J., Cyr D. M., Douglas M. G. Eukaryotic homologues of Escherichia coli dnaJ: a diverse protein family that functions with hsp70 stress proteins. Mol Biol Cell. 1993 Jun;4(6):555–563. doi: 10.1091/mbc.4.6.555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chappell T. G., Konforti B. B., Schmid S. L., Rothman J. E. The ATPase core of a clathrin uncoating protein. J Biol Chem. 1987 Jan 15;262(2):746–751. [PubMed] [Google Scholar]
- Chiang H. L., Terlecky S. R., Plant C. P., Dice J. F. A role for a 70-kilodalton heat shock protein in lysosomal degradation of intracellular proteins. Science. 1989 Oct 20;246(4928):382–385. doi: 10.1126/science.2799391. [DOI] [PubMed] [Google Scholar]
- Chirico W. J., Waters M. G., Blobel G. 70K heat shock related proteins stimulate protein translocation into microsomes. Nature. 1988 Apr 28;332(6167):805–810. doi: 10.1038/332805a0. [DOI] [PubMed] [Google Scholar]
- Cyr D. M., Lu X., Douglas M. G. Regulation of Hsp70 function by a eukaryotic DnaJ homolog. J Biol Chem. 1992 Oct 15;267(29):20927–20931. [PubMed] [Google Scholar]
- DeLuca-Flaherty C., McKay D. B., Parham P., Hill B. L. Uncoating protein (hsc70) binds a conformationally labile domain of clathrin light chain LCa to stimulate ATP hydrolysis. Cell. 1990 Sep 7;62(5):875–887. doi: 10.1016/0092-8674(90)90263-e. [DOI] [PubMed] [Google Scholar]
- Dworniczak B., Mirault M. E. Structure and expression of a human gene coding for a 71 kd heat shock 'cognate' protein. Nucleic Acids Res. 1987 Jul 10;15(13):5181–5197. doi: 10.1093/nar/15.13.5181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flaherty K. M., DeLuca-Flaherty C., McKay D. B. Three-dimensional structure of the ATPase fragment of a 70K heat-shock cognate protein. Nature. 1990 Aug 16;346(6285):623–628. doi: 10.1038/346623a0. [DOI] [PubMed] [Google Scholar]
- Flajnik M. F., Canel C., Kramer J., Kasahara M. Which came first, MHC class I or class II? Immunogenetics. 1991;33(5-6):295–300. doi: 10.1007/BF00216688. [DOI] [PubMed] [Google Scholar]
- Flynn G. C., Chappell T. G., Rothman J. E. Peptide binding and release by proteins implicated as catalysts of protein assembly. Science. 1989 Jul 28;245(4916):385–390. doi: 10.1126/science.2756425. [DOI] [PubMed] [Google Scholar]
- Gao Y., Thomas J. O., Chow R. L., Lee G. H., Cowan N. J. A cytoplasmic chaperonin that catalyzes beta-actin folding. Cell. 1992 Jun 12;69(6):1043–1050. doi: 10.1016/0092-8674(92)90622-j. [DOI] [PubMed] [Google Scholar]
- Giebel L. B., Dworniczak B. P., Bautz E. K. Developmental regulation of a constitutively expressed mouse mRNA encoding a 72-kDa heat shock-like protein. Dev Biol. 1988 Jan;125(1):200–207. doi: 10.1016/0012-1606(88)90073-5. [DOI] [PubMed] [Google Scholar]
- Haus U., Trommler P., Fisher P. R., Hartmann H., Lottspeich F., Noegel A. A., Schleicher M. The heat shock cognate protein from Dictyostelium affects actin polymerization through interaction with the actin-binding protein cap32/34. EMBO J. 1993 Oct;12(10):3763–3771. doi: 10.1002/j.1460-2075.1993.tb06054.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt C., Calderwood S. Characterization and sequence of a mouse hsp70 gene and its expression in mouse cell lines. Gene. 1990 Mar 15;87(2):199–204. doi: 10.1016/0378-1119(90)90302-8. [DOI] [PubMed] [Google Scholar]
- Hunt C., Morimoto R. I. Conserved features of eukaryotic hsp70 genes revealed by comparison with the nucleotide sequence of human hsp70. Proc Natl Acad Sci U S A. 1985 Oct;82(19):6455–6459. doi: 10.1073/pnas.82.19.6455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingolia T. D., Craig E. A. Drosophila gene related to the major heat shock-induced gene is transcribed at normal temperatures and not induced by heat shock. Proc Natl Acad Sci U S A. 1982 Jan;79(2):525–529. doi: 10.1073/pnas.79.2.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang P. J., Ostermann J., Shilling J., Neupert W., Craig E. A., Pfanner N. Requirement for hsp70 in the mitochondrial matrix for translocation and folding of precursor proteins. Nature. 1990 Nov 8;348(6297):137–143. doi: 10.1038/348137a0. [DOI] [PubMed] [Google Scholar]
- Laloraya S., Gambill B. D., Craig E. A. A role for a eukaryotic GrpE-related protein, Mge1p, in protein translocation. Proc Natl Acad Sci U S A. 1994 Jul 5;91(14):6481–6485. doi: 10.1073/pnas.91.14.6481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis V. A., Hynes G. M., Zheng D., Saibil H., Willison K. T-complex polypeptide-1 is a subunit of a heteromeric particle in the eukaryotic cytosol. Nature. 1992 Jul 16;358(6383):249–252. doi: 10.1038/358249a0. [DOI] [PubMed] [Google Scholar]
- Liberek K., Marszalek J., Ang D., Georgopoulos C., Zylicz M. Escherichia coli DnaJ and GrpE heat shock proteins jointly stimulate ATPase activity of DnaK. Proc Natl Acad Sci U S A. 1991 Apr 1;88(7):2874–2878. doi: 10.1073/pnas.88.7.2874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liberek K., Skowyra D., Zylicz M., Johnson C., Georgopoulos C. The Escherichia coli DnaK chaperone, the 70-kDa heat shock protein eukaryotic equivalent, changes conformation upon ATP hydrolysis, thus triggering its dissociation from a bound target protein. J Biol Chem. 1991 Aug 5;266(22):14491–14496. [PubMed] [Google Scholar]
- Milarski K. L., Morimoto R. I. Expression of human HSP70 during the synthetic phase of the cell cycle. Proc Natl Acad Sci U S A. 1986 Dec;83(24):9517–9521. doi: 10.1073/pnas.83.24.9517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milarski K. L., Morimoto R. I. Mutational analysis of the human HSP70 protein: distinct domains for nucleolar localization and adenosine triphosphate binding. J Cell Biol. 1989 Nov;109(5):1947–1962. doi: 10.1083/jcb.109.5.1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montgomery D., Jordan R., McMacken R., Freire E. Thermodynamic and structural analysis of the folding/unfolding transitions of the Escherichia coli molecular chaperone DnaK. J Mol Biol. 1993 Jul 20;232(2):680–692. doi: 10.1006/jmbi.1993.1418. [DOI] [PubMed] [Google Scholar]
- Morimoto R. I., Hunt C., Huang S. Y., Berg K. L., Banerji S. S. Organization, nucleotide sequence, and transcription of the chicken HSP70 gene. J Biol Chem. 1986 Sep 25;261(27):12692–12699. [PubMed] [Google Scholar]
- Morimoto R. I., Sarge K. D., Abravaya K. Transcriptional regulation of heat shock genes. A paradigm for inducible genomic responses. J Biol Chem. 1992 Nov 5;267(31):21987–21990. [PubMed] [Google Scholar]
- Munro S., Pelham H. R. An Hsp70-like protein in the ER: identity with the 78 kd glucose-regulated protein and immunoglobulin heavy chain binding protein. Cell. 1986 Jul 18;46(2):291–300. doi: 10.1016/0092-8674(86)90746-4. [DOI] [PubMed] [Google Scholar]
- Murakami H., Pain D., Blobel G. 70-kD heat shock-related protein is one of at least two distinct cytosolic factors stimulating protein import into mitochondria. J Cell Biol. 1988 Dec;107(6 Pt 1):2051–2057. doi: 10.1083/jcb.107.6.2051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson R. J., Ziegelhoffer T., Nicolet C., Werner-Washburne M., Craig E. A. The translation machinery and 70 kd heat shock protein cooperate in protein synthesis. Cell. 1992 Oct 2;71(1):97–105. doi: 10.1016/0092-8674(92)90269-i. [DOI] [PubMed] [Google Scholar]
- O'Malley K., Mauron A., Barchas J. D., Kedes L. Constitutively expressed rat mRNA encoding a 70-kilodalton heat-shock-like protein. Mol Cell Biol. 1985 Dec;5(12):3476–3483. doi: 10.1128/mcb.5.12.3476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostermann J., Horwich A. L., Neupert W., Hartl F. U. Protein folding in mitochondria requires complex formation with hsp60 and ATP hydrolysis. Nature. 1989 Sep 14;341(6238):125–130. doi: 10.1038/341125a0. [DOI] [PubMed] [Google Scholar]
- Palleros D. R., Reid K. L., Shi L., Welch W. J., Fink A. L. ATP-induced protein-Hsp70 complex dissociation requires K+ but not ATP hydrolysis. Nature. 1993 Oct 14;365(6447):664–666. doi: 10.1038/365664a0. [DOI] [PubMed] [Google Scholar]
- Palleros D. R., Welch W. J., Fink A. L. Interaction of hsp70 with unfolded proteins: effects of temperature and nucleotides on the kinetics of binding. Proc Natl Acad Sci U S A. 1991 Jul 1;88(13):5719–5723. doi: 10.1073/pnas.88.13.5719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raabe T., Manley J. L. A human homologue of the Escherichia coli DnaJ heat-shock protein. Nucleic Acids Res. 1991 Dec 11;19(23):6645–6645. doi: 10.1093/nar/19.23.6645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Requena J. M., López M. C., Jimenez-Ruiz A., de la Torre J. C., Alonso C. A head-to-tail tandem organization of hsp70 genes in Trypanosoma cruzi. Nucleic Acids Res. 1988 Feb 25;16(4):1393–1406. doi: 10.1093/nar/16.4.1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rochester D. E., Winer J. A., Shah D. M. The structure and expression of maize genes encoding the major heat shock protein, hsp70. EMBO J. 1986 Mar;5(3):451–458. doi: 10.1002/j.1460-2075.1986.tb04233.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadis S., Hightower L. E. Unfolded proteins stimulate molecular chaperone Hsc70 ATPase by accelerating ADP/ATP exchange. Biochemistry. 1992 Oct 6;31(39):9406–9412. doi: 10.1021/bi00154a012. [DOI] [PubMed] [Google Scholar]
- Shi Y., Thomas J. O. The transport of proteins into the nucleus requires the 70-kilodalton heat shock protein or its cytosolic cognate. Mol Cell Biol. 1992 May;12(5):2186–2192. doi: 10.1128/mcb.12.5.2186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skowyra D., Georgopoulos C., Zylicz M. The E. coli dnaK gene product, the hsp70 homolog, can reactivate heat-inactivated RNA polymerase in an ATP hydrolysis-dependent manner. Cell. 1990 Sep 7;62(5):939–944. doi: 10.1016/0092-8674(90)90268-j. [DOI] [PubMed] [Google Scholar]
- Slater M. R., Craig E. A. The SSA1 and SSA2 genes of the yeast Saccharomyces cerevisiae. Nucleic Acids Res. 1989 Jan 25;17(2):805–806. doi: 10.1093/nar/17.2.805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith D. F., Faber L. E., Toft D. O. Purification of unactivated progesterone receptor and identification of novel receptor-associated proteins. J Biol Chem. 1990 Mar 5;265(7):3996–4003. [PubMed] [Google Scholar]
- Stone D. E., Craig E. A. Self-regulation of 70-kilodalton heat shock proteins in Saccharomyces cerevisiae. Mol Cell Biol. 1990 Apr;10(4):1622–1632. doi: 10.1128/mcb.10.4.1622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilly K., McKittrick N., Zylicz M., Georgopoulos C. The dnaK protein modulates the heat-shock response of Escherichia coli. Cell. 1983 Sep;34(2):641–646. doi: 10.1016/0092-8674(83)90396-3. [DOI] [PubMed] [Google Scholar]
- Ting J., Lee A. S. Human gene encoding the 78,000-dalton glucose-regulated protein and its pseudogene: structure, conservation, and regulation. DNA. 1988 May;7(4):275–286. doi: 10.1089/dna.1988.7.275. [DOI] [PubMed] [Google Scholar]
- Tsai M. Y., Wang C. Uncoupling of peptide-stimulated ATPase and clathrin-uncoating activity in deletion mutant of hsc70. J Biol Chem. 1994 Feb 25;269(8):5958–5962. [PubMed] [Google Scholar]
- Ungewickell E. The 70-kd mammalian heat shock proteins are structurally and functionally related to the uncoating protein that releases clathrin triskelia from coated vesicles. EMBO J. 1985 Dec 16;4(13A):3385–3391. doi: 10.1002/j.1460-2075.1985.tb04094.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu B., Hunt C., Morimoto R. Structure and expression of the human gene encoding major heat shock protein HSP70. Mol Cell Biol. 1985 Feb;5(2):330–341. doi: 10.1128/mcb.5.2.330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaffe M. B., Farr G. W., Miklos D., Horwich A. L., Sternlicht M. L., Sternlicht H. TCP1 complex is a molecular chaperone in tubulin biogenesis. Nature. 1992 Jul 16;358(6383):245–248. doi: 10.1038/358245a0. [DOI] [PubMed] [Google Scholar]
- Zylicz M., Yamamoto T., McKittrick N., Sell S., Georgopoulos C. Purification and properties of the dnaJ replication protein of Escherichia coli. J Biol Chem. 1985 Jun 25;260(12):7591–7598. [PubMed] [Google Scholar]









