Figure 2.
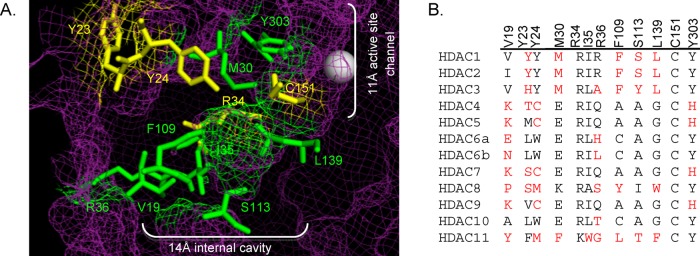
(A) Amino acid residues in the 14 Å cavity of HDAC1 that are mutated in this study (yellow and green, ball and stick structures) are highlighted in the HDAC1 crystal structure (shown as purple mesh, 4BKX). The metal ion required for catalysis is shown as a gray sphere. (B) Catalytic domains of the class I, II, and IV human HDAC proteins were aligned (ClustalW), and residues located in the 14 Å internal cavity are shown. Residues differing from the most highly conserved at each position are highlighted in red. The numbering at the top is for HDAC1. (Genbank accession numbers: HDAC1, Q13547.1; HDAC2, Q92769.2; HDAC3, NP_003874.2; HDAC4, AAD29046.1; HDAC5, AAD29047.1; HDAC6, AAD29048.1; HDAC7, NP_056216.2; HDAC8, CAB90213.1; HDAC9, AAK66821.1; HDAC10, NP_114408.3; and HDAC11, NP_079103.2.)
