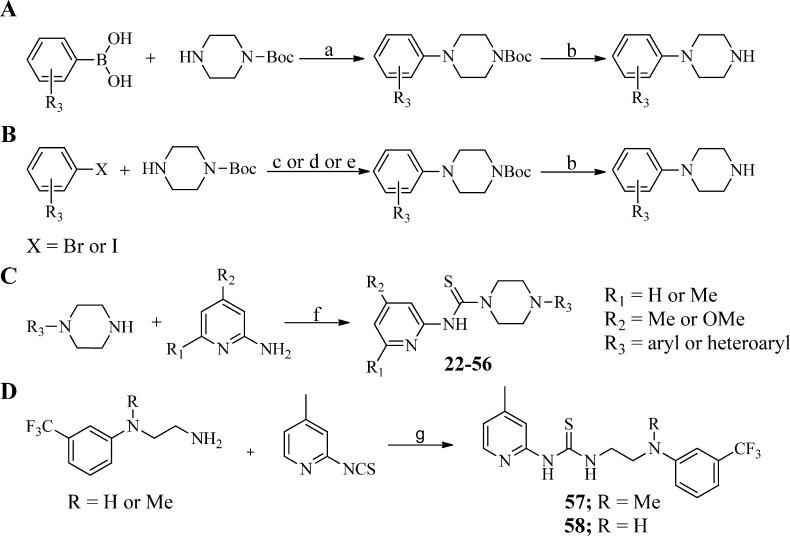
Scheme 3. Synthesis of Requisite Aryl/Heteroarylpiperazines and Analogues 22–58.
Reagents and conditions: (a) Cu(OAc)2 (10 mol %), 4 Å molecular sieves, O2, CH2Cl2, 45 °C, 12–24 h; (b) TFA/CH2Cl2, rt, 1 h; (c) BINAP (10 mol %), Pd(OAc)2 (5 mol %), Cs2CO3 (1.5 equiv), toluene, 110 °C, 12–24 h; (d) JohnPhos (10 mol %), Pd2(dba)3 (5 mol %), t-BuONa (1.5 equiv), toluene, 110 °C, 2–8 h; (e) BINOL (20 mol %), CuBr (20 mol %), K3PO4 (2 equiv), DMF, rt, 12–24 h; (f) 1,1′-thiocarbonyldiimidazole (TCDI), CH2Cl2, 40 °C; (g) DMF, 90 °C, 1 h.