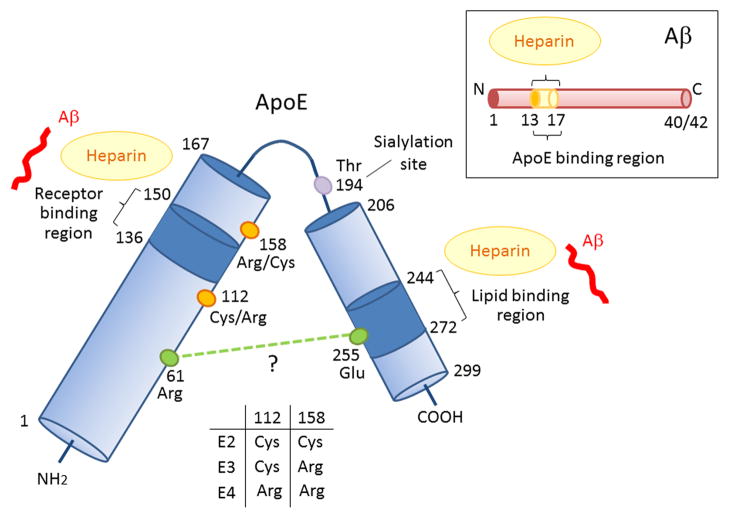
Figure 1. Schematic illustration of structural and functional regions of apoE and Aβ.
Human apoE is a glycosylated protein of 299 amino acids consisting of a receptor-binding region (residues 136–150) in the N-terminal domain (residues 1–167) and a lipid-binding region (residues 244–272) in the C-terminal domain (residues 206–299) (Chen et al., 2011). ApoE also has two heparin-binding sites, each within the N-terminal and the C-terminal domains (Ji et al., 1993; Saito et al., 2003). The residues that distinguish the apoE isoforms are located at residues 112 and 158, where apoE2 has Cys residues at both positions, apoE3 has a Cys residue at 112 and an Arg residue at 158, and apoE4 has Arg residues at both positions (Rall et al., 1982; Weisgraber et al., 1981). The domain interaction between Arg 61 and Glu 255 in apoE4 is also indicated (Mahley and Rall, 2000; Mahley et al., 2009; Wilson et al., 1991), although other structural studies have not confirmed this (Chen et al., 2011). Aβ can interact with both the receptor-binding region and lipid-binding region of apoE, as well as with heparin through its residues 13–17 (Strittmatter et al., 1993; Winkler et al., 1999).