Table 2. Structures and Activities of 3k Variants, Exploring Bioisosteric Replacements and Scaffold Perturbation.
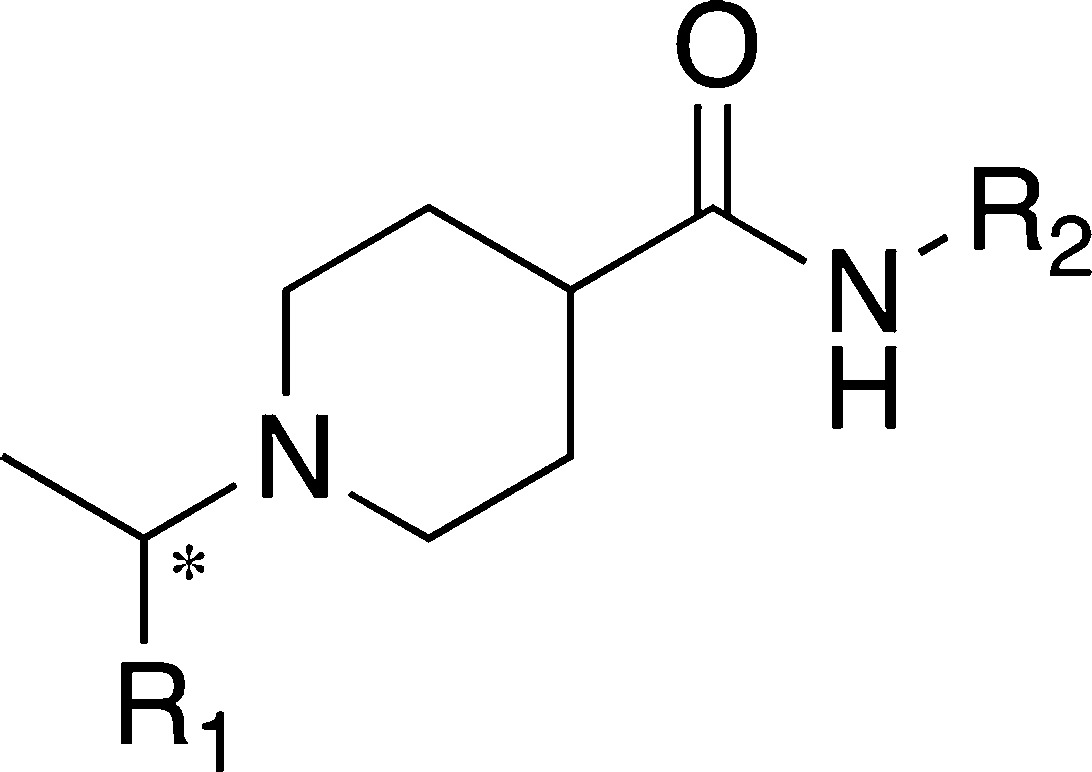
| compd | isomera | R1 | R2 | IC50 (μM)b |
|---|---|---|---|---|
| 3k | R | 1-naphthyl | 3-F-Ph-CH2 | 0.15 ± 0.01 |
| 4a | R,S | 8-quinolinyl | 3-F-Ph-CH2 | 7.0 ± 0.7 |
| 4b | R,S | 5-quinolinyl | 3-F-Ph-CH2 | 4.5 ± 0.2 |
| 4c | R,S | 5-isoquinolinyl | 3-F-Ph-CH2 | 6.8 ± 0.3 |
| 4d | R,S | 1-isoquinolinyl | 3-F-Ph-CH2 | 30.8 ± 2.6 |
| 5a | R | 1-naphthyl | 3-pyridinyl-CH2 | 26.3 ± 2.3 |
| 5b | R | 1-naphthyl | 4-pyridinyl-CH2 | 18.3 ± 0.9 |
| 5c | R | 1-naphthyl | 2-methoxy-4-pyridinyl-CH2 | 0.35 ± 0.02 |
| 6a | R | 1-naphthyl | 4-Cl-Ph-CH2CH2 | 1.6 ± 0.3 |
| 6b | R | 1-naphthyl | 3-F-Ph-CH2CH2 | 1.9 ± 0.1 |
Chiral center.
Values are reported as mean ± standard deviation based on a minimum of triplicate measurements.
