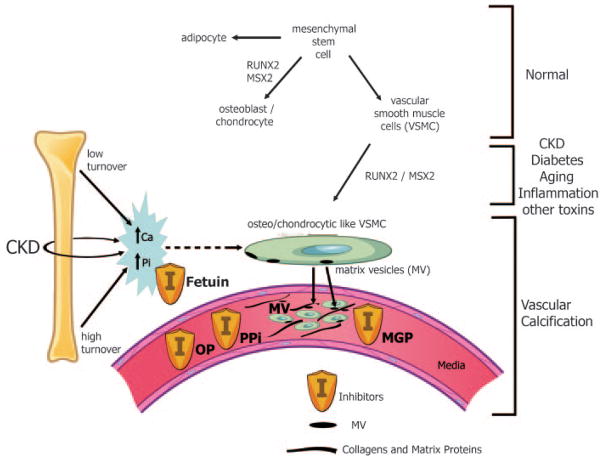
Figure 5. Pathogenesis of Vascular Calcification.
Normally, mesenchymal stem cells differentiate to adipocytes, osteoblasts, chondrocytes, and vascular smooth muscle cells (VSMC). In the setting of CKD, diabetes, aging, inflammation, and multiple other toxins, these VSMC can dedifferentiate or transform into osteo/chondrocytic-like cells by upregulation of transcription factors such as RUNX-2 and MSX2. These transcription factors are critical for normal bone development and thus their upregulation in VSMC is indicative of a phenotypic switch. These osteo/chondrocytic-like VSMC then become calcified in a process similar to bone formation. These cells lay down collagen and noncollagenous proteins in the intima or media and incorporate calcium and phosphorus into matrix vesicles to initiate mineralization and further mineralize into hydroxyapatite. The overall positive calcium and phosphorus balance of most dialysis patients feeds both the cellular transformation and the generation of matrix vesicles. In addition, the extremes of bone turnover in chronic kidney disease (low and high or adynamic and hyperparathyroid bone, respectively) will increase the available calcium and phosphorus by altering the bone content of these minerals. Ultimately, whether an artery calcifies or not depends on the strength of the army of inhibitors (I) standing by in the circulation (fetuin A) and in the arteries (PPI = pyrophosphate, MGP = matrix Gla protein, and OP = osteopontin as examples). Reproduced with permission of the American Society of Nephrology from Figure 1 in Moe et al. J Am Soc Nephrol. 2008;19:213–216.