Fig. 6.
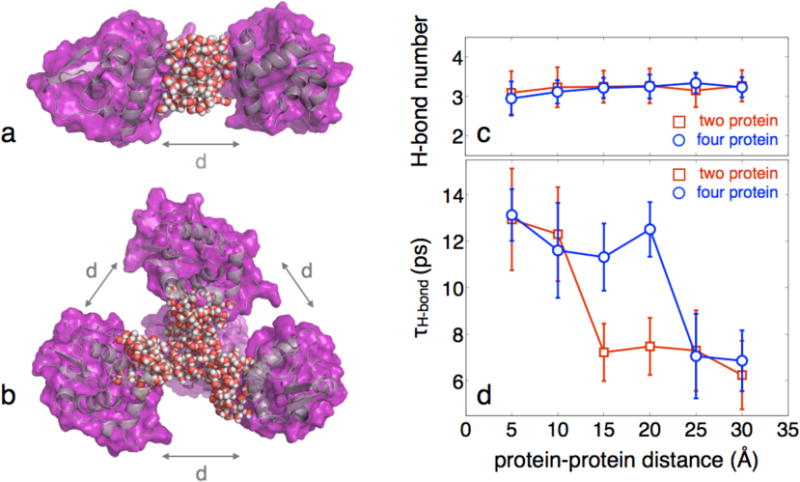
Example of the simulation analysis where (a) two proteins are separated by a set distance d and the bridging water is selected for analysis and (b) four proteins are arranged tetrahedrally, all of which are separated by the same variable distance. The water that was selected for analysis is shown. c, Hydrogen bond number of the crowded water as a function of protein-protein distance. In each case there is no clear transition in the average hydrogen bonds per water molecule, suggesting no significant change in structure. A slight downward trend is observed as the interprotein distance is reduced, though this is the result of a higher relative contribution from the interfacial water, which has fewer hydrogen bonds than bulk water. d, Hydrogen bond correlation times of the crowded water as a function of protein-protein distance. The occurrence of a dynamic transition is found between 10-15 Å for two proteins and 20-25 Å for the four protein simulation. In each case, only a weak coupling is observed before and after the dynamic transition. The results not only demonstrate a percolation-like transition of water dynamics upon crowding, but also show that the distance of this transition is a function of the degree and geometry of crowding.
