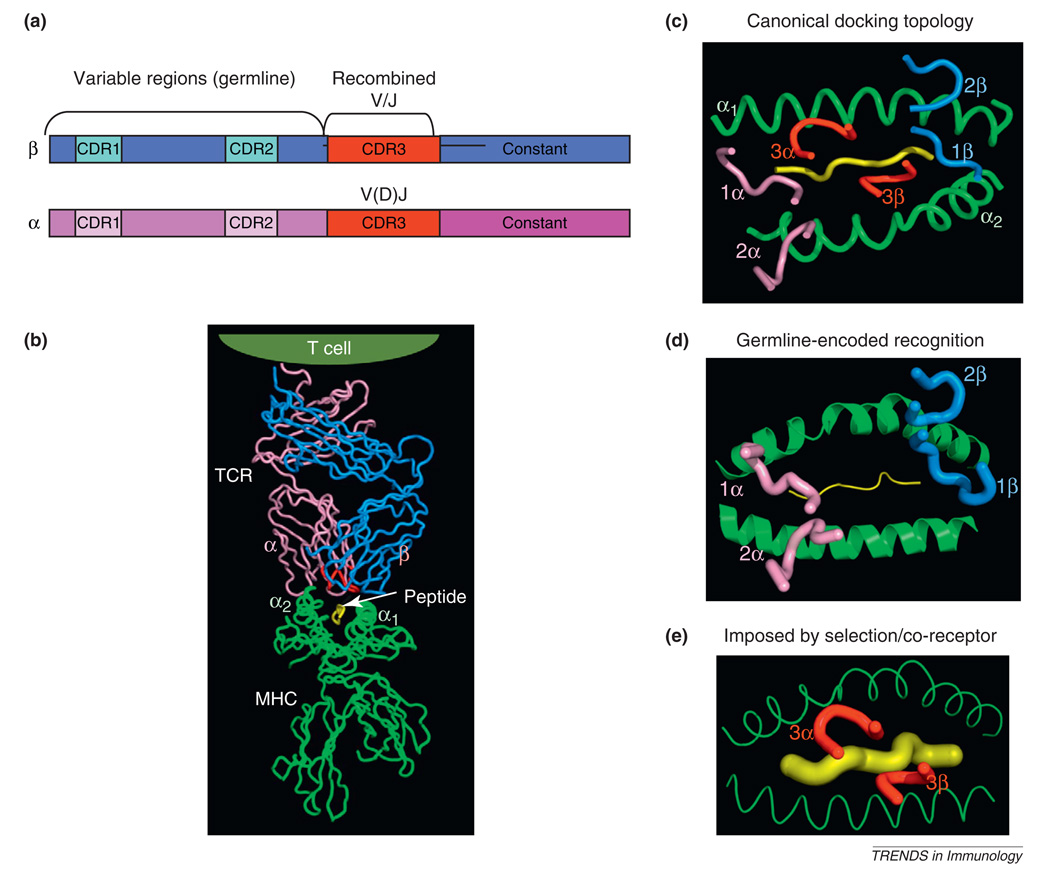
Figure 1.
Structural models of T cell receptor (TCR) recognition of peptide–MHC. (a) Schematic of TCR α and β chain genes showing segregation of the germline and recombined regions. (b) Structure of an αβ TCR bound to a class I MHC presenting a peptide antigen [56]. The CDR3 loops in the TCR binding site are colored red. (c) Classical ‘footprint’ view of the CDR loops of the TCR overlaying the peptide and MHC. This view shows the roughly diagonal binding orientation with the germline-derived CDRs 1 and 2 primarily engaging the MHC helices, and the TCR CDR3s engaging the peptide. The angular orientation of these CDR loops to the long axis of the MHC groove is referred to as the docking angle or docking topology. (d) The germline hypothesis is indicated by the primacy of the CDR1 and CDR2 germline derived TCR loops forming the principal interactions with the MHC independently of CDR3. (e) In contrast to (d), the alternative model proposes that the CDR3–peptide interaction, or the influence of co-receptor during thymic selection, underlies TCR–MHC specificity irrespective of germline influences.