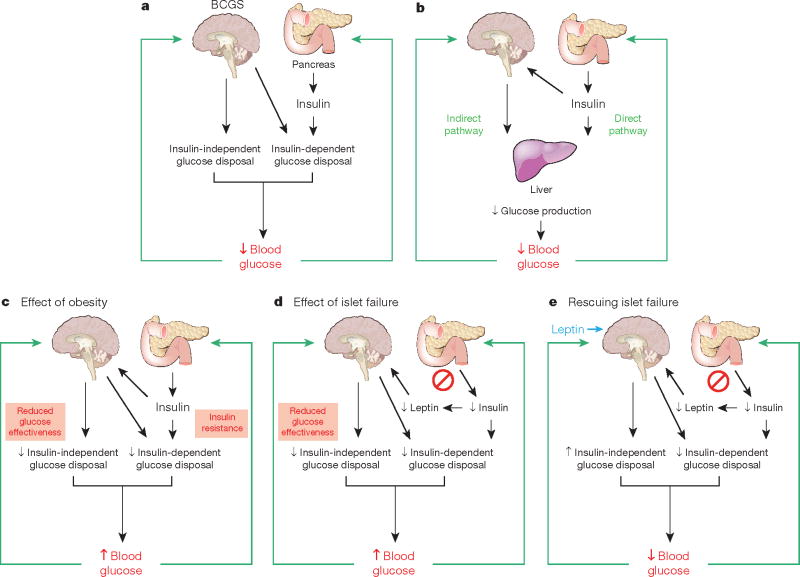
Box 2 Figure. Model integrating the BCGS and islet-centred system in normal and abnormal glucose homeostasis.
a, Under normal conditions, glucose homeostasis is controlled by complex and highly coordinated interactions between brain- and islet-centred systems. Like islets, the BCGS senses a variety of humoral signals, and in response to these inputs, BCGS activation increases glucose disposal by both insulin-dependent (for example, by increasing tissue insulin sensitivity) and insulin-independent (by increasing GE, which accounts for ∼50% of overall glucose disposal39) mechanisms. b, Although insulin normally inhibits HGP through its direct action on the liver, an indirect pathway also exists through which insulin can preserve normal HGP and blood glucose levels even when hepatocytes cannot respond to insulin directly25. We propose that this is among the effects mediated by the BCGS. c, Obesity is associated with reduced GE39 and with insulin resistance, and BCGS dysfunction contributes to both. When BCGS dysfunction is mild, the resulting tendency for blood glucose levels to increase stimulates insulin secretion, such that glucose homeostasis is preserved (at the expense of higher insulin levels). When BCGS dysfunction is more severe, however, even marked hyperinsulinaemia cannot preserve normal glucose homeostasis35, owing in part to the inability of reduced GE to be compensated by increased insulin secretion. Thus, intact BCGS function is required for normal glucose homeostasis. d, Islet dysfunction is not compensated by BCGS activation; to the contrary, impaired islet function can itself impair BCGS function (by reducing secretion of leptin as well insulin, when islet damage is severe) creating a vicious cycle that results initially in glucose intolerance. As both BCGS and islet dysfunction progress, overt hyperglycaemia and T2D result. e, Islet dysfunction can be compensated for by supraphysiological BCGS activation, which can achieve near-normal glucose homeostasis in rodent models of diabetes via insulin-independent mechanisms. Thus, therapeutic interventions targeting the BCGS as well as the traditional islet-based system may achieve diabetes remission, whereas targeting just one system typically does not.