Abstract
Objectives
In a trial completed in 2010, U.S. patients with diabetes and depression (DMD) were randomized to usual care or telephone cognitive behavioral therapy (CBT) that emphasized physical activity. 12-month intervention effects were observed for blood pressure, depression, and pedometer-measured step-counts. The current study examined variation in intervention effects across patient subgroups defined by a measure of clinical complexity.
Methods
Three groups of patients were identified at baseline using the Vector Model of Complexity that recognizes socioeconomic, biological, behavioral, and other determinants of treatment response. Complexity-by-intervention interactions were examined using regression models.
Results
Intervention effects for blood pressure, depression, and step-counts differed across complexity levels (each p<.01). Effects on Beck Depression Inventory (BDI) scores were greater in the low-complexity group (-8.8) than in the medium- (-3.2) or high-complexity groups (-2.7). Physical activity effects also were greatest in the low-complexity group (increase of 1,498 steps/day). In contrast, systolic blood pressure effects were greater among intervention patients with high complexity (-8.5 mmHg).
Conclusions
This intervention had varying impacts on physical and mental health depending on patients' clinical complexity. Physical activity and depressive symptom gains may be more likely among less complex patients, although more complex patients may achieve cardiovascular benefits through decreased blood pressures.
Keywords: diabetes, depression, care management, telehealth, comorbidity, physical activity
Introduction
Depression is a common comorbidity for patients with diabetes, and patients with diabetes plus depression (DMD) have poorer diabetes outcomes than other diabetes patients.1-6 Self-care behaviors, including physical activity, have been clearly linked to depressive symptomotology.7-11 Cognitive behavioral therapy (CBT) may be a particularly useful approach to improving outcomes for DMD patients, because CBT can treat patients' depressive symptoms directly as well as address the diabetes-specific cognitions that are important mediators of effective self-management.12 These collateral benefits of CBT are particularly important, since diabetes-related distress may be one of the correlates of depressive symptoms that is most directly related to poor glycemic control.13 Given the myriad of short-term and long-term management goals for DMD patients, interventions may be especially beneficial when they focus on behaviors that influence both patients' mood as well as their risk for micro- and macrovascular complications. Physical activity is an important behavioral target because it can improve patients' depressive symptoms, glycemic control, and other cardiovascular risk factors.12,14-20 Another advantage of physical activity-focused interventions is that pedometers can be a behavioral motivator and also provide an objective measure for gauging the impact of the program.21
The “Positive Steps Study” is a recently completed, multi-site randomized trial comparing the outcomes of enhanced usual care to a telephone-based program of CBT that emphasized the links between depression and diabetes as well as the promotion of physical activity. A separate paper 22 reports that among participants the mean baseline level for A1c (the primary outcome for the trial) was 7.6%, and there was no notable differences in A1c across groups at 12 months. However, the intervention had statistically significant positive impacts at 12 months on patients' systolic blood pressures (mean relative reduction of 4.26 mmHg), depressive symptoms as measured by the Beck Depression Inventory (mean relative reduction of 4.5 points), and pedometer-measured step-counts (mean relative increase of 1,131 steps per day; all p< .05).
While these average intervention effects are encouraging, the benefits of this intervention may not have been experienced equally across all patients in the study. In particular, patients' clinical complexity may affect their ability to make behavioral changes or benefit physiologically from the intervention. Complexity is a multi-dimensional construct, reflecting the socioeconomic, cultural, biological, environmental, and behavioral characteristics that can interact with patients' treatment and determine outcomes.23,24 Evidence suggests that outcomes of diabetes management can be influenced by comorbidities and other factors that increase complexity.25-27
A complexity measure may be useful for identifying subgroups of clinical trial participants who are more or less likely to benefit than average. However, researchers often evaluate variation in intervention effects by creating multiple subgroups defined by baseline levels of each outcome, as well as potentially arbitrary patient characteristics such as age, gender, and race. Hayward and colleagues 28 have pointed out that this approach capitalizes on chance variations, complicates interpretability (i.e., because patients are differently distributed across groups for each analysis) and could obscure important intervention benefits among patients with the greatest overall need for additional services. Subgroup analysis based on a multidimensional complexity score avoids these problems and may uncover groups most likely to benefit from a new program, while providing clues regarding mechanisms of intervention effect.
The purpose of the current study was to re-examine differences in effects on outcomes (blood pressure, depressive symptoms, physical activity, and A1c) among participants in the Positive Steps Study across groups representing varying levels of clinical complexity at the time of entry into the trial.
Methods
Overview of the Design
This was a secondary analysis of data collected as part of the Positive Steps Study. In brief, patients with diabetes and depression were randomized to enhanced usual care or telephone cognitive behavioral therapy which emphasized the links between depression and diabetes as well as a physical activity program with pedometers. Endpoints, including A1c, blood pressure, depression scores, and pedometer-measured step-counts were measured at 12-months post enrollment. Detailed methods are described elsewhere22 and summarized below.
Recruitment and Randomization
Adult participants with diabetes using antihyperglycemic medication were identified from a community-based non-profit healthcare system, a university healthcare system, and a VA healthcare system. Patients were ineligible if at the time of telephone screening they had a Patient Health Questionnaire-9 (PHQ-9)29 depression score of < 11, had been diagnosed with bipolar disorder or schizophrenia, were diagnosed with diabetes before age 15 and only using insulin (i.e., likely type 1 patients), or were in active treatment for another life-threatening condition. Additional patients were excluded if they reported a recent change in their depression management or that they were unable to walk either one block or 10 minutes without rest.
Eligible patients were invited to an in-person screening and recruitment visit. Patients were excluded if: they had a Beck Depression Inventory (BDI)30 score < 14, they scored < 21 on the Short Orientation Memory Concentration Test,31 or they reported active drug or alcohol abuse using the CAGE questionnaire.32 All patients completed a written informed consent. The study was approved by the IRB in each of the three participating health care systems. Enrolled patients were randomized with equal probability to either the intervention or usual care using sealed opaque envelopes and a table of random numbers.
Intervention
Intervention patients participated in a 12-month telephone CBT program delivered by registered nurses with a mix of psychiatric and primary care training. The CBT program included an initial intensive phase of 12-weekly sessions followed by nine monthly booster sessions. At first, CBT focused exclusively on patients' depressive symptoms; after five sessions, nurse counselors introduced concepts related to a pedometer-based walking program, and the links between depression, physical activity, and diabetes outcomes.
Prior to initiating patient counseling, each nurse participated in an intensive training program including a six-session CBT course. Nurses also participated in weekly group supervisory sessions, where they discussed problematic cases and shared information about strategies for completing the CBT protocol. Each nurse audio-recorded initial sessions with their patients, and those recordings were reviewed by an experienced CBT supervisor and trainer during group supervision. Both nurses and patients used a week-by-week manual to guide their CBT sessions. Nurse manuals included check-lists for each week's CBT goals. During each session, nurses monitored patients' depressive symptoms using the PHQ and monitored their activity levels using the PASE.29,33 Patient manuals included logs that patients could use to complete CBT homework exercises and to monitor their progress toward step-count goals.
Enhanced Usual Care
Usual care patients received: a copy of the Feeling Good Handbook - a self-help book based on cognitive behavioral therapy for depression,34 National Institute of Mental Health educational materials about depression, educational materials about walking and diabetes, and a list of local resources for depression. If usual care patients allowed, their primary care physician was notified about their depression scores.
Measurement
Patients completed in-person interviews at baseline and 12-months. Interviews were conducted by trained, non-clinical research assistants. At both time points, their A1c was measured using the DCA2000® point-of-care analyzer.35 Blood pressure was measured in both arms using an Omron® automatic monitor with a repeat measurement in the arm with the highest pressure after several minutes of rest. This third measure was used in study analyses.
Six weeks after completing their baseline assessment, all patients were sent an Omron® HJ-720 ITC pedometer. Pedometers were sent blinded using a removable sticker, and patients were instructed to wear the pedometer throughout waking hours for seven consecutive days. At the end of the week, patients were instructed to remove the sticker and contact the study team to report their step-counts. Those who did not call the team were contacted by research staff. Control-group patients then returned the pedometer; intervention patients were instructed to keep the pedometer for use in their walking program. At the 12 month follow-up, all control patients as well as intervention patients who had lost their pedometer were again sent a blinded pedometer with similar instructions.
The main depression measure was the Beck Depression Inventory (BDI).30 Perceived self-efficacy for physical activity and diet were measured using the Perceived Competence Scale.36,37 Adherence to antihyperglycemic medication was measured using the Morisky medication adherence scale.38
Baseline Complexity Levels
Patients' clinical complexity at baseline was measured using the Vector Control Model of Complexity.23 Much like the Andersen and Aday model for treatment access,39 the Vector model articulates dimensions of complexity that can be measured differently depending on the specific purpose of the study and available data. In this study, we used the following indicators for each dimension of complexity: socioeconomic: age 75 or higher, high school education or less, annual income $10,000 or less; culture: non-white race or Hispanic ethnicity; biologic/genetic: Body Mass Index of 40 or higher (Class III Obesity), high A1c (i.e., 8.0% or higher if the patient was less than 65 years of age and 8.5% or higher if age 65 or older), systolic blood pressure 140 mmHg or higher (indicating uncontrolled hypertension), BDI depression score greater than 28 (indicating moderate or severe depression); environmental/ecological: no spouse or other partner; behavioral: diabetes medication adherence score greater than 2 on the Morisky scale,38 no evidence of at least moderate physical activity (less than 2000 pedometer steps per day or missing baseline pedometer data), dietary and activity-related self-efficacy in the lowest quartile of the distribution on the Perceived Competence Scale.36,37 Patients received one point for each indicator. The resulting score ranged from 0-12 and was collapsed into three roughly equal groups based on tertiles of the distribution representing low-complexity (scores of 0 or 1), medium-complexity (score=2-3), and high-complexity (4+) patients.
Analysis
The current study was based on new models of complexity emerging since the trial's design as well as feedback from intervention nurses regarding the role of comorbidity as an impediment to patients' ability to respond to the new service provided. As such, the hypothesis tested was not planned a priori and the results are exploratory.
In initial analyses, variation in patients' baseline characteristics was examined across complexity levels and then across experimental groups within complexity levels. Outcomes of interest included the primary outcome for the trial (A1c), and secondary outcomes that were shown to have an overall average intervention effect (systolic blood pressure, BDI depression score, and step-counts). To identify potential complexity-by-intervention interactions, regression models were fit predicting each endpoint value controlling for baseline values. Those models included terms designed to identify variation in intervention effects across complexity subgroups by including dummy variables for combinations of complexity group and treatment arm (i.e., medium complexity+control, high complexity+ control, low complexity+intervention, medium complexity+intervention, and high complexity+intervention). Post-fitting statistical tests compared the magnitude of estimated coefficients for intervention effects within complexity levels in order to identifying significant intervention/control differences within strata. A global statistical test was conducted to identify an overall variation in intervention effects across complexity levels. These models and baseline values for each outcome within the three complexity levels were used to calculate predicted post-intervention values controlling for baseline differences and secular trends. Finally, regression models were re-calculated after converting each outcome to a z-score so that the varying patterns in the relationship between receipt of the intervention and complexity could be illustrated graphically.
Results
Baseline Characteristics of the Sample
A total of 5,542 patients were identified from electronic records, of which 474 (8.6%) were found to be eligible during telephone screening. Of those patients, 51 did not attend the in-person eligibility screening and 84 were found to be ineligible at that subsequent screening. The remaining 339 patients were enrolled in the trial, of whom 291 (86%) provided A1c, blood pressure, and survey data at the 12-month follow-up. Patients who failed to provide these follow-up data were similar on a large number of measures (including all outcomes presented here) but were somewhat more satisfied at baseline with their healthcare (p<.05). Of those providing in-person follow-up data, 214 (74%) provided both baseline and follow-up pedometer data. Those without step-count data had lower incomes, higher systolic blood pressures, and higher (i.e., worse) BDI scores at baseline.
For the overall sample, there were no significant differences at baseline in intervention and control patients' sociodemographic characteristics, A1cs, blood pressures, depressive symptoms, or survey-based outcomes. Patients' mean age was 56 years, half were women, and 84% were White. The mean baseline A1c in the overall sample was 7.6% (SD: 1.7).
When classifying patients according to baseline complexity level, 25% of the sample was classified as low complexity, 40% as medium complexity, and 35% as high complexity. Individual health and behavioral risk factors varied systematically across complexity levels (Table 1). As expected, high-complexity patients were very sedentary, with 73.5% having an average of less than 2000 steps per day (as compared to the low complexity group with only 11.1% this sedentary). Moreover, more than half of high complexity patients had: BMI's > 40, systolic blood pressures > 140 mmHg, BDI depression scores > 28, no spousal partner, and low diabetes self-care self-efficacy scores. Baseline values for each outcome were similar across treatment groups within complexity strata (Table 2).
Table 1. Baseline Characteristics by Complexity Level.
| Low | Medium | High | p-value | |
|---|---|---|---|---|
| N | 72 | 117 | 102 | |
| Socioeconomic | ||||
| Age > 75 | 5.6 (4) | 22.2 (26) | 25.5 (26) | 0.003 |
| ≤ High School Education | 6.9 (5) | 31.6 (37) | 49.3 (50) | <.001 |
| Annual Income < $10,000 | 6.9 (5) | 22.2 (26) | 49.3 (50) | <.001 |
| Culture | ||||
| Black/Hispanic/Other Race | 5.6 (4) | 13.7 (16) | 25.5 (26) | 0.001 |
| Biological/genetic | ||||
| Body Mass Index > 40 | 12.5 (9) | 23.1 (27) | 52.0 (53) | <.001 |
| High A1c(a) | 6.9 (5) | 17.1 (20) | 29.4 (30) | 0.001 |
| Systolic BP > 140 mmHg | 18.1 (13) | 34.2 (40) | 55.9 (57) | <.001 |
| BDI Depression Score > 28(b) | 15.3 (11) | 34.2 (40) | 56.9 (58) | <.001 |
| Environmental/Ecological | ||||
| No Spouse/Partner | 27.8 (20) | 35.0 (41) | 58.5 (60) | <.001 |
| Behavioral | ||||
| Poor Adherence to DM Rx (c) | 1.4 (1) | 5.1 (6) | 22.6 (23) | <0.001 |
| < 2000 Steps per day | 11.1 (8) | 53.0 (62) | 73.5 (75) | <.001 |
| Low Self-Care Self-Efficacy(d) | 29.2 (21) | 53.9 (63) | 70.6 (72) | <.001 |
Note: Cell entries are column percent (N)
(a) > 8% if age < 65 and > 8.5% if age 65+
(b) Indicates moderate/severe depression
(c) Morisky adherence score > 2
(d) Lowest quartile on the Perceived Competence Scale
Table 2. Baseline Values for Outcomes within Groups Defined by Clinical Complexity.
| Low (N=72) | Medium (N=117) | High (N=102) | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| CBT | Control | CBT | Control | CBT | Control | |
| N | 43 | 29 | 56 | 61 | 46 | 56 |
| Systolic BP (mmHg) | 129.8 ± 2.4 | 129.2 ± 2.7 | 134.1 ± 2.2 | 132.1 ± 2.2 | 144.1 ± 2.4 | 138.1 ± 2.1 |
| Diastolic BP (mmHg) | 78.1 ± 1.4 | 80.7 ± 2.0 | 79.5 ± 1.4 | 78.1 ± 1.3 | 81.8 ± 1.6 | 80.7 ± 1.6 |
| Steps (a) | 4336 ± 327 | 4734 ± 568 | 2783 ± 267 | 2871 ± 245 | 2449 ± 305 | 2431 ± 333 |
| BDI Depression | 25.1 ± 1.1 | 21.9 ± 1.5 | 26.1 ± 1.1 | 25.6 ± 1.2 | 29.0 ± 1.0 | 29.8 ± 1.4 |
| % A1c | 7.0 ± 0.1 | 7.3 ± 0.3 | 7.5 ± 0.2 | 7.4 ± 0.2 | 7.9 ± 0.3 | 8.2 ± 0.3 |
Notes: Cell entries are mean ± SE
No mean differences within complexity strata were statistically significant (p<.05).
(a) 214 patients with other outcome data provided data on baseline and follow-up step-counts. N's of CBT and Control patients with stepcount data were as follows: Low complexity: 27 and 37, Medium complexity: 50 and 34, High complexity: 42 and 24.
Variation in Intervention Effects across Complexity Levels
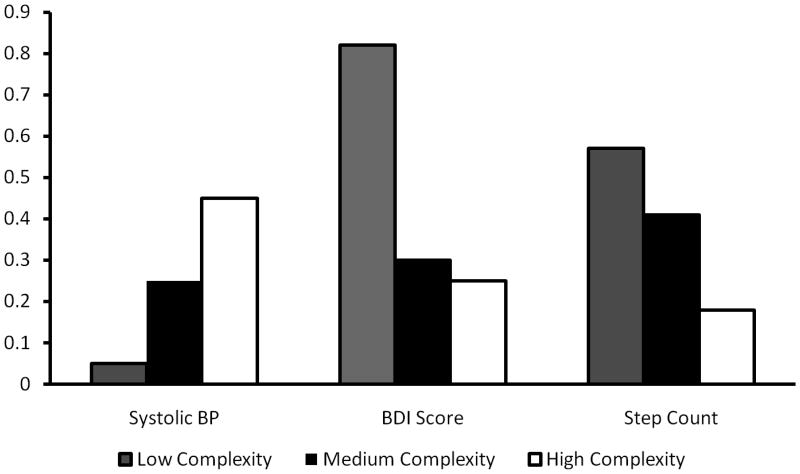
Heterogeneity of treatment effect across complexity levels was not detected for the trial's primary outcome, A1c, but was detected for the three secondary outcomes that showed a significant main treatment effects: systolic blood pressure, step-counts, and BDI depression scores (Table 3). Regression models identified large and statistically significant intervention effects on systolic blood pressure among high-complexity patients, but not among low-complexity patients. An isolated reduction in diastolic blood pressures that was not found in the main trial analysis was identified in the high complexity group. In contrast, the largest impacts on step-counts and BDI scores were observed in the lowest-complexity stratum. The figure illustrates the significant variation in effects across complexity strata (Figure).
Table 3. Variation in Intervention Effects Across Complexity Levels.
| Low (N=72) | Medium (N=117) | High ((N=102) | Interaction | ||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| CBT | Control | CBT | Control | CBT | Control | p-value(c) | |
| N | 43 | 29 | 56 | 61 | 46 | 56 | |
| Systolic BP (mmHg) | 0.004 | ||||||
| Predicted Value | 128.3 | 127.4 | 128.2 | 132.7 | 131.8 | 140.4 | |
| Intervention Effect | 0.9 | -4.5 | -8.5(b) | ||||
| Diastolic BP (mmHg) | 0.01 | ||||||
| Predicted Value | 78.4 | 78.0 | 75.6 | 76.9 | 75.9 | 80.2 | |
| Intervention Effect | 0.5 | -1.4 | -4.3(b) | ||||
| Steps(a) | <.001 | ||||||
| Predicted Value | 5988 | 4490 | 3983 | 2896 | 3503 | 3054 | |
| Intervention Effect | 1498(b) | 1087(b) | 449 | ||||
| BDI Depression | <.001 | ||||||
| Predicted Value | 9.0 | 17.8 | 15.0 | 18.2 | 16.9 | 19.7 | |
| Intervention Effect | -8.8(b) | -3.2 | -2.7 | ||||
| A1c | 0.12 | ||||||
| Predicted Value | 7.21 | 7.55 | 7.81 | 7.45 | 8.21 | 8.12 | |
| Intervention Effect | -0.3 | 0.4 | 0.1 | ||||
Note: In MANOVA models including terms for randomization group and complexity level, intervention groups were significantly different with respect to systolic BP, diastolic BP, step counts, and BDI. Predicted endpoint values were calculated based on regression models that controlled for differences in baseline values and secular trends.
(a) 214 patients with other outcome data provided data on baseline and follow-up ste-counts. N's of CBT and Control patients with ste-count data were as follows: Low complexity 27 and 37, Medium complexity: 50 and 34, High complexity: 42 and 24.
(b) Statistically significant (p<.05) intervention group differences within that complexity stratum.
(c) p-value represents the probability that the intervention effect was equivalent across complexity levels.
Figure.
Variation in standardized effect scores across complexity levels. Positive standardized scores for each outcome represent improvements in health. BDI depression scores decreased the most among intervention patients in the low complexity group while step-counts increased the most in that group. In contrast, systolic blood pressures decreased the most in the high-complexity group.
Discussion
In this randomized trial with overall intervention effects on DMD patients' systolic blood pressures, pedometer-measured step counts, and BDI depression scores, we found that those effects varied significantly depending on the patient's clinical complexity upon entry into the trial. Specifically, depressive symptoms and physical activity levels improved the most in the low-complexity group, while the overall average improvement in systolic blood pressure was driven by improvements in pressures among the most complex intervention participants.
The association between decreased depressive symptoms and increased step-counts for low complexity patients likely reflects multiple, reciprocal processes. Cross-sectional studies show that depressive symptoms and activity levels correlate.40 Lack of motivation and sedentary behavior are common symptoms of depression and improvements in mood would be expected to increase physical activity.41 On the other hand, depression interventions are less effective in increasing physical activity,42 in part because effective depression treatment often neglects behavioral strategies for physical activity reinforcement.
Among both medium and high-complexity patients, we found that step-count increases were modest. This lack of response to the walking component of the intervention is disappointing, but perhaps not surprising. Maintaining a physical activity program over the course of a year is difficult for most people,43 and is particularly difficult for those with multiple comorbidities, very high BMI's, and other factors contributing to clinical complexity. Older individuals frequently list medical problems as the primary barrier to participation in physical activity programs.44 This pedometer-based walking program was designed specifically for multi-morbid patients45 and such programs have been found to be effective for patients with a variety of chronic conditions.46-48 However, the current study suggests that among patients who all have at minimum two serious comorbid chronic diseases (i.e., diabetes and depression), relatively complex patients may face such serious physical barriers to walking (e.g., pain and deconditioning) that more intensive clinician-guided interventions are required.25,49
It is unclear why high complexity patients experienced a substantial improvement in their systolic blood pressures, despite relatively small improvements in physical activity and depression scores. Of course, because patients in this group had relatively high pressures at baseline, one might expect more improvement; however this same pattern was not observed for step counts or BDI depression scores. The two most direct strategies for improving blood pressures are ensuring that patients are prescribed the appropriate pharmacotherapy and that they take those medications as prescribed. Unfortunately, the current study did not collect data specifically on antihypertensive regimen changes or medication adherence. Given the large impacts on systolic pressures observed and the clinical importance of such improvements in hypertension management, future studies should explore similar interventions for complex patients as well as the mechanisms of action for treatment effects.
As with any secondary analysis of randomized trial results, these findings are exploratory. Patients were not randomized within complexity levels and the study was not powered a priori for testing intervention-control differences within those levels. Although baseline intervention-control differences were small within complexity strata and baseline values were controlled for in all outcome analyses, it is possible that unmeasured differences in baseline health status across groups could affect the result reported here. In the US, the majority of people who receive a clinical diagnosis of diabetes are put on antihyperglycemic medication either at the time of diagnosis or within the subsequent six month. The current study applies only to that population. It should be noted that physical activity also is an important behavior for improving cardiovascular health and preventing conversion to diabetes among pre-diabetic patients.50 Finally, as with all subgroup analyses, multiple-comparisons may contribute to the findings and they should be interpreted as hypothesis generating rather than definitive.
Conclusions
In sum, we found significant heterogeneity of treatment effects for the Positive Steps intervention according to patients' baseline complexity levels. The least complex patients benefited the most with respect to their depression and physical inactivity, while more complex patients experienced significant improvements in their systolic blood pressures, despite less improvement in these former two outcomes. While telehealth management interventions such as this can improve blood pressures among highly complex patients, more intensive interventions may be required to address those patients' persistent depressive symptoms and very low levels of physical activity. Complexity represents a broad array of determinants, and capturing those data through systematic assessment or clinical interview could be useful in tailoring interventions to patients most likely to benefit. For researchers, analyzing trial results according to a priori subgroups that vary in terms of clinical complexity could ensure that important intervention benefits are not missed in overall average effects driven by a large number of program participants who may not respond to treatment. Novel services for patients with diabetes and other chronic conditions should move beyond the “one-size-fits-all approach” in order to develop a portfolio of evidence-based options that fit with patients' needs, resources, and preferences.51
Acknowledgments
This study was supported by NIH grant # 5R18DK66166-3, the Michigan Diabetes Research and Training Center (NIH #DK020572) and the Michigan Institute for Clinical and Health Research (NIH #UL1RR024986). John Piette is a VA Senior Research Career Scientist. At the time of the study, Caroline Richardson was supported by NIH training grant # K23 HL075098. Dana Striplin, M.P.H., managed all data collection and participated in data analysis. Her effort was supported by NIH grant # 5R18DK66166-3. John W. Williams, M.D. contributed important expert advice during the development of the intervention and the trial design.
This NIH-funded trial (1R18DK066166-01A1) is registered with ClinicalTrials.gov and the registration number is NCT01106885.
Footnotes
Conflicts of Interest: No authors have any conflict of interest (financial or material support or assistance) related to this manuscript in any area, including the design and conduct of the study; collection, management, analysis, and interpretation of the data; or preparation, review, and approval of the final version of the paper.
References
- 1.Lustman PJ, Anderson RJ, Freedland KE, de Groot M, Carney RM, Clouse RE. Depression and poor glycemic control: a meta-analytic review of the literature. Diabetes Care. 2000;23(7):934–942. doi: 10.2337/diacare.23.7.934. [DOI] [PubMed] [Google Scholar]
- 2.Ciechanowski PS, Katon WJ, Russo JE. Depression and diabetes: impact of depressive symptoms on adherence, function, and costs. Arch Intern Med. 2000 Nov 27;160(21):3278–3285. doi: 10.1001/archinte.160.21.3278. [DOI] [PubMed] [Google Scholar]
- 3.Bogner HR, Morales KH, Post EP, Bruce ML. Diabetes, depression, and death: a randomized controlled trial of a depression treatment program for older adults based in primary care (PROSPECT) Diabetes Care. 2007 Dec;30(12):3005–3010. doi: 10.2337/dc07-0974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Subramaniam M, Sum CF, Pek E, et al. Comorbid depression and increased health care utilisation in individuals with diabetes. Gen Hosp Psychiatry. 2009 May-Jun;31(3):220–224. doi: 10.1016/j.genhosppsych.2009.01.001. [DOI] [PubMed] [Google Scholar]
- 5.de Groot M, Anderson R, Freedland KE, Clouse RE, Lustman PJ. Association of depression and diabetes complications: a meta-analysis. Psychosom Med. 2001 Jul-Aug;63(4):619–630. doi: 10.1097/00006842-200107000-00015. [DOI] [PubMed] [Google Scholar]
- 6.Katon WJ, Rutter C, Simon G, et al. The association of comorbid depression with mortality in patients with type 2 diabetes. Diabetes Care. 2005 Nov;28(11):2668–2672. doi: 10.2337/diacare.28.11.2668. [DOI] [PubMed] [Google Scholar]
- 7.Kilbourne AM, Reynolds CF, 3rd, Good CB, Sereika SM, Justice AC, Fine MJ. How does depression influence diabetes medication adherence in older patients? Am J Geriatr Psychiatry. 2005 Mar;13(3):202–210. doi: 10.1176/appi.ajgp.13.3.202. [DOI] [PubMed] [Google Scholar]
- 8.Lin EH, Katon W, Von Korff M, et al. Relationship of depression and diabetes self-care, medication adherence, and preventive care. Diabetes Care. 2004 Sep;27(9):2154–2160. doi: 10.2337/diacare.27.9.2154. [DOI] [PubMed] [Google Scholar]
- 9.Gonzalez JS, Peyrot M, McCarl LA, et al. Depression and diabetes treatment nonadherence: a meta-analysis. Diabetes Care. 2008 Dec;31(12):2398–2403. doi: 10.2337/dc08-1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Katon WJ, Russo JE, Heckbert SR, et al. The relationship between changes in depression symptoms and changes in health risk behaviors in patients with diabetes. Int J Geriatr Psychiatry. 2010 May;25(5):466–475. doi: 10.1002/gps.2363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Richardson LK, Egede LE, Mueller M, Echols CL, Gebregziabher M. Longitudinal effects of depression on glycemic control in veterans with Type 2 diabetes. Gen Hosp Psychiatry. 2008 Nov-Dec;30(6):509–514. doi: 10.1016/j.genhosppsych.2008.07.001. [DOI] [PubMed] [Google Scholar]
- 12.Piette JD, Richardson C, Valenstein M. Addressing the needs of patients with multiple chronic illnesses: the case of diabetes and depression. Am J Manag Care. 2004;10(2):41–51. [PubMed] [Google Scholar]
- 13.Fisher L, Mullan JT, Arean P, Glasgow RE, Hessler D, Masharani U. Diabetes distress but not clinical depression or depressive symptoms is associated with glycemic control in both cross-sectional and longitudinal analyses. Diabetes Care. 2010 Jan;33(1):23–28. doi: 10.2337/dc09-1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moreau KL, Degarmo R, Langley J, et al. Increasing daily walking lowers blood pressure in postmenopausal women. Med Sci Sports Exerc. 2001;33(11):1825–1831. doi: 10.1097/00005768-200111000-00005. [DOI] [PubMed] [Google Scholar]
- 15.Lysy Z, Da Costa D, Dasgupta K. The association of physical activity and depression in Type 2 diabetes. Diabet Med. 2008 Oct;25(10):1133–1141. doi: 10.1111/j.1464-5491.2008.02545.x. [DOI] [PubMed] [Google Scholar]
- 16.Iwane M, Arita M, Tomimoto S, et al. Walking 10,000 steps/day or more reduces blood pressure and sympathetic nerve activity in mild essential hypertension. Hypertens Res. 2000 Nov;23(6):573–580. doi: 10.1291/hypres.23.573. [DOI] [PubMed] [Google Scholar]
- 17.McKercher CM, Schmidt MD, Sanderson KA, Patton GC, Dwyer T, Venn AJ. Physical activity and depression in young adults. Am J Prev Med. 2009 Feb;36(2):161–164. doi: 10.1016/j.amepre.2008.09.036. [DOI] [PubMed] [Google Scholar]
- 18.Armstrong K, Edwards H. The effectiveness of a pram-walking exercise programme in reducing depressive symptomatology for postnatal women. Int J Nurs Pract. 2004 Aug;10(4):177–194. doi: 10.1111/j.1440-172X.2004.00478.x. [DOI] [PubMed] [Google Scholar]
- 19.Merom D, Phongsavan P, Wagner R, et al. Promoting walking as an adjunct intervention to group cognitive behavioral therapy for anxiety disorders--a pilot group randomized trial. J Anxiety Disord. 2008 Aug;22(6):959–968. doi: 10.1016/j.janxdis.2007.09.010. [DOI] [PubMed] [Google Scholar]
- 20.Suija K, Pechter U, Kalda R, Tahepold H, Maaroos J, Maaroos HI. Physical activity of depressed patients and their motivation to exercise: Nordic Walking in family practice. Int J Rehabil Res. 2009 Jun;32(2):132–138. doi: 10.1097/MRR.0b013e32831e44ef. [DOI] [PubMed] [Google Scholar]
- 21.Bravata DM, Smith-Spangler C, Sundaram V, et al. Using pedometers to increase physical activity and improve health: a systematic review. Jama. 2007 Nov 21;298(19):2296–2304. doi: 10.1001/jama.298.19.2296. [DOI] [PubMed] [Google Scholar]
- 22.Piette JD, Richardson C, Himle J, et al. A randomized trial of telephone counseling plus walking for depressed diabetes patients. Medical Care. doi: 10.1097/MLR.0b013e318215d0c9. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Safford MM, Allison JJ, Kiefe CI. Patient complexity: more than comorbidity. The Vector Model of Complexity Journal of General Internal Medicine. 2007;22(Suppl 3):382–390. doi: 10.1007/s11606-007-0307-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Safford MM, Brmacombe M, Zhang Q, et al. Patient complexity in quality comparisons for glycemic control: an observational study. Implementation Science. 2009;4(2):1–8. doi: 10.1186/1748-5908-4-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Krein SL, Heisler M, Piette JD, Makki F, Kerr EA. The effect of chronic pain on diabetes patients' self-management. Diabetes Care. 2005;28(1):65–70. doi: 10.2337/diacare.28.1.65. [DOI] [PubMed] [Google Scholar]
- 26.Zulman DM, Kerr EA, Hofer TP, Heisler M, Zikmund-Fisher BJ. Patient-provider concordance in the prioritization of health conditions among hypertensive diabetes patients. Journal of General Internal Medicine. 2010;25(5):408–414. doi: 10.1007/s11606-009-1232-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kerr EA, Zikmund-Fisher B, Klamerus ML, Subramanian U, Hogan MM, Hofer TP. The role of clinical uncertainty in treatment decisions for diabetic patients with uncontrolled blood pressure. Annals of Internal Medicine. 2008;148(10):783–785. doi: 10.7326/0003-4819-148-10-200805200-00004. [DOI] [PubMed] [Google Scholar]
- 28.Hayward RA, Kent DM, Vijan S, Hofer TP. Reporting clinical trial results to inform providers, payers, and consumers. Health Affairs. 2005;24(6):1571–1581. doi: 10.1377/hlthaff.24.6.1571. [DOI] [PubMed] [Google Scholar]
- 29.Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16(9):606–613. doi: 10.1046/j.1525-1497.2001.016009606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Beck A, Ward C, Mendelson M, Mock J, Earbaugh K. An inventory for measuring depression. Arch Gen Psychiatry. 1961;4:561–571. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- 31.Katzman R, Brown T, Fuld P, Peck A, Schechter R, Schimmel H. Validation of a short orientation-memory-concentration test of cognitive impairment. Am J Psychiatry. 1983;140(6):734–739. doi: 10.1176/ajp.140.6.734. [DOI] [PubMed] [Google Scholar]
- 32.Midanik LT. Perspectives on the validity of self-reported alcohol use. Br J Addict. 1989;84(12):1419–1423. doi: 10.1111/j.1360-0443.1989.tb03920.x. [DOI] [PubMed] [Google Scholar]
- 33.Washburn RA, McAuley E, Katula J, Mihalko SL, Boileau RA. The physical activity scale for the elderly (PASE): evidence for validity. J Clin Epidemiol. 1999;52(7):643–651. doi: 10.1016/s0895-4356(99)00049-9. [DOI] [PubMed] [Google Scholar]
- 34.Burns DD. The feeling good handbook. New York, NY: Plume Book; 1989. [Google Scholar]
- 35.Arsie MP, Marchioro L, Lapolla A, et al. Evaluation of diagnostic reliability of DCA 2000 for rapid and simple monitoring of HbA1c. Acta Diabetologica. 2000;37(1):1–7. doi: 10.1007/s005920070028. [DOI] [PubMed] [Google Scholar]
- 36.Williams GC, Freedman ZR, Deci EL. Supporting autonomy to motivate patients with diabetes for glucose control. Diabetes Care. 1998;21(10):1644–1651. doi: 10.2337/diacare.21.10.1644. [DOI] [PubMed] [Google Scholar]
- 37.Williams GC, Freedman ZR, Deci EL. Internalization of biopsychosocial values by medical students: a test of self-determination theory. Journal of Personality and Social Psychology. 1996;70:767–779. doi: 10.1037//0022-3514.70.4.767. [DOI] [PubMed] [Google Scholar]
- 38.Morisky DE, Green LW, Levine DM. Concurrent and predictive validity of a self-reported measure of medication adherence. Med Care. 1986;24(1):67–74. doi: 10.1097/00005650-198601000-00007. [DOI] [PubMed] [Google Scholar]
- 39.Andersen RM, McCutcheon A, Aday LA, Chiu GY, Bell R. Exploring dimensions of access to medical care. Health Services Research. 1983;18(1):49–73. [PMC free article] [PubMed] [Google Scholar]
- 40.Strine TW, Mokdad AH, Dube SR, et al. The association of depression and anxiety with obesity and unhealthy behaviors among community-dwelling US adults. General Hospital Psychiatry. 2008;30(2):127–137. doi: 10.1016/j.genhosppsych.2007.12.008. [DOI] [PubMed] [Google Scholar]
- 41.Dunn AL, Trivedi MH, Kampert JB, Clark CG, Chambliss HO. Exercise treatment for depression: efficacy and dose response. American Journal of Preventive Medicine. 2005;28(1):1–8. doi: 10.1016/j.amepre.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 42.Lin EH, Katon W, Rutter C, et al. Effects of enhanced depression treatment on diabetes self-care. Annals of Family Medicine. 2006;4(1):46–53. doi: 10.1370/afm.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dishman RK, Buckworth J. Increasing physical activity: a quantitative synthesis. Medicine & Science in Sports and Exercise. 1996;28(6):706–719. doi: 10.1097/00005768-199606000-00010. [DOI] [PubMed] [Google Scholar]
- 44.Chinn DJ, White J, Harland J, Drinkwater C, Raybould S. Barriers to physical activity and socioeconomic position: implications for health promotion. Journal of Epidemiology and Community Health. 1999;53(3):191–192. doi: 10.1136/jech.53.3.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Moy ML, Janney AW, Ngyuen HQ, et al. Use of a pedometer and Internet-mediated walking program in patients with chronic obstructive pulmonary disease. Journal of Rehabilitation Research and Development. 2010;47(5):485–496. doi: 10.1682/jrrd.2009.07.0091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Furber S, Monger C, Franco L, et al. The effectiveness of a brief intervention using a pedometer and step-recording diary in promoting physical activity in people diagnosed with type 2 diabetes or impaired glucose tolerance. Health Promotion Journal of Australia. 2008;19(3):189–195. doi: 10.1071/he08189. [DOI] [PubMed] [Google Scholar]
- 47.Vallance JK, Courneya KS, Plotnikoff RC, Yasui Y, Mackey JR. Randomized controlled trial of the effects of print materials and step pedometers on physical activity and quality of life in breast cancer survivors. Journal of Clinical Oncology. 2007;25(17):2352–2359. doi: 10.1200/JCO.2006.07.9988. [DOI] [PubMed] [Google Scholar]
- 48.Weinstock RS, Brooks G, Palmas W, et al. Lessened decline in physical activity and impairment of older adults with diabetes with telemedicine and pedometer use: results from the IDEATel Study. Age and Aging. 2010 doi: 10.1093/ageing/afq147. epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 49.Krein SL, Heisler M, Piette JD, Butchart A, Kerr EA. Overcoming the influence of chronic pain on older patients' difficulty with recommended self-management activities. Gerontologist. 2007 Feb 1;47(1):61–68. doi: 10.1093/geront/47.1.61. 2007. [DOI] [PubMed] [Google Scholar]
- 50.Knowler WC, Fowler SE, Hamman RF, et al. 10-year follow-up of diabetes incidence and weight loss in the Diabetes Prevention Program Outcomes Study. Lancet. 2009;374(9702):1677–1686. doi: 10.1016/S0140-6736(09)61457-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Piette J. Interactive behavior change technology to support diabetes self-management: where do we stand? Diabetes Care. 2007;30(10):2425–2432. doi: 10.2337/dc07-1046. [DOI] [PubMed] [Google Scholar]