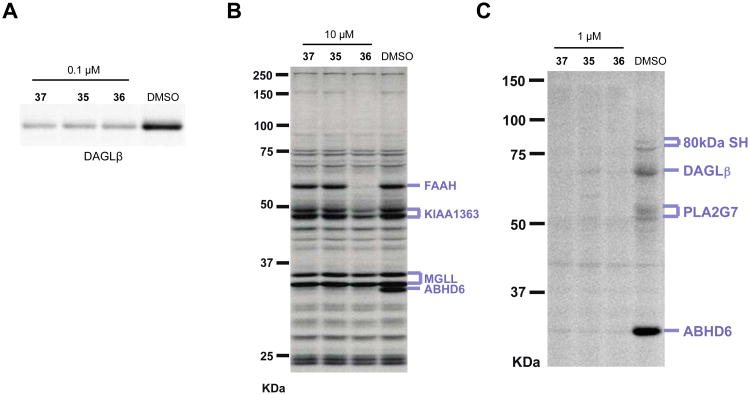
Figure 5.
Potency and selectivity of acyclic phenethyl-1,2,3-TUs. (A) Inhibitory activity of compounds (0.1 μM) against recombinant DAGLβ expressed by transient transfect in HEK293T cells (DAGLβ-HEK293T lysates) as determined by gel-based competitive ABPP using 38 probe. (B, C) Selectivity of compounds against mouse brain SHs as measured by gel-based competitive ABPP using FP-Rh (B) or 38 (C). For the gel-based ABPP assays, proteomes were incubated with compound (0.1, 1, or 10 μM) for 30 min at 37 °C followed by reaction with fluorescent ABPP probes (38 or FP-Rh, 1 μM, 30 min, 37 °C). Fluorescent gel images are shown in gray scale. Serine hydrolase activities in gels were assigned as described in Figure 2.