Table 1.
Comparison of activity of (2-benzyl)-Pip-heterocyclic urea derivatives.
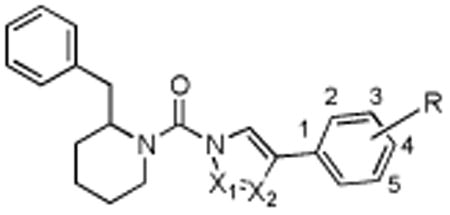
| |||
|---|---|---|---|
|
| |||
| Structure | Compound | DAGLβ % inhibition (100 nM) | Off-targets (10 μM) |
| X1 = N, X2 = N, R = 4-OCF3 | 5 | 52 | FAAH, ABHD6, ABHD11, PLA2G7 |
| X1 = N, X2 = C, R = 4-OCF3 | 8 | 0 | none |
| X1 = C, X2 = N, R = 4-OCF3 | 9 | 0 | none |
| X1 = N, X2 = N, R = 3-5 diF | 10 | 55.4 | FAAH, KIAA1363, ABHD6, LYPLA1, LYPLA2, PLA2G7, ABHD11 |
| X1 = N, X2 = N, R = 4-Ph | 11 (KT109) | 60.8 | ABHD6, PLA2G7 |
| X1 = N, X2 = N, R = 3-Ph | 14 | 27.4 | FAAH, MGLL, ABHD6, 80 kDa SH, ABHD11 |
| X1 = N, X2 = N, R = 2-Ph | 15 | 17.6 | FAAH, MGLL, ABHD6, ABHD11 |
| X1 = N, X2 = N, R = 4-piperidineamide | 19 | 40.9 | FAAH, ABHD6, ABHD11 |
