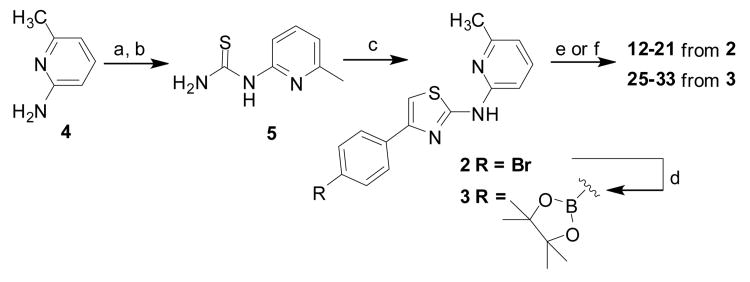
Scheme 1.
Synthesis of key synthetic intermediates 2 and 3 used in subsequent coupling reactions to prepare 12–21 and 25–33. Reaction conditions: (a) BzSCN, acetone, reflux; (b) NaOH, MeOH, reflux; (c) bromoacetophenone, EtOH, reflux; (d) bis(pinacolato)diboron, Pd (dppf)2Cl2, AcOK, dioxane, 80 °C. (e) For compounds 12-21 from 2: ArB(OH)2, 1,4-dioxane, K3PO4, Pd2(dba)3, tricyclohexylphosphine, microwave (150 °C, 30 min); (f) For compounds 25-33 from 3: ArBr, Pd(dppf)2Cl2, K2CO3, 1,4-dioxane/water (5:1), 80 °C, overnight.