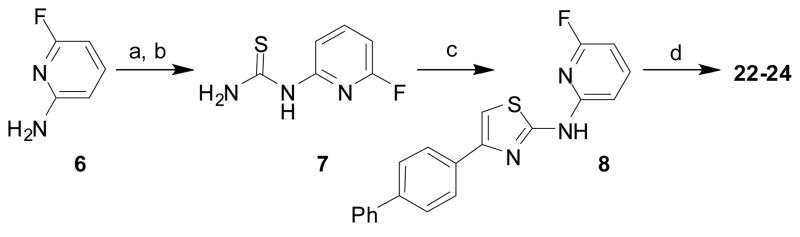
Scheme 2.
Synthesis of key synthetic intermediate 8 used in subsequent SNAr reactions to prepare analogs 22–24. Reaction conditions: (a) BzNCS, acetone, reflux; (b) NaOH, MeOH, reflux; (c) 1-([1,1′-biphenyl]-4-yl)-2-bromoethanone, NaHCO3, CH3CN, reflux; (d) ROH and Na or R1R2NH, 120-130 °C, 4-12 hr.