Table 1.
Antiprion potency for AMT analogs.
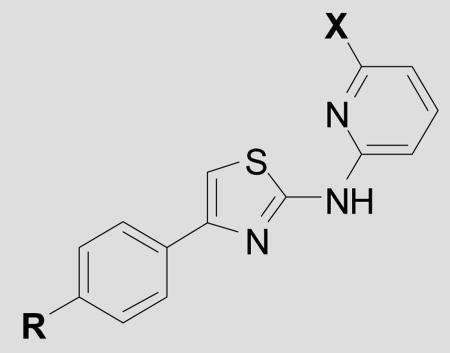
| ||||
|---|---|---|---|---|
| Cmpd | R = | X = | EC50 ± SEM(nM)[a] | n[B] |
| 1 | Phenyl | CH3 | 1290 ± 122 | 85 |
| 12 | pyridin-4-yl | CH3 | 87 ± 43 | 3 |
| 13 | pyridin-4-yl N-oxide | CH3 | 559 ± 120 | 3 |
| 14 | 2-Me-pyridin-4-yl | CH3 | 400 ± 61 | 3 |
| 15 | pyridin-3-yl | CH3 | 68 ± 13 | 5 |
| 16 | pyridin-3-yl N-oxide | CH3 | 203 ± 10 | 3 |
| 17 | 2-Me-pyridin-3-yl | CH3 | 618 ± 54 | 3 |
| 18 |
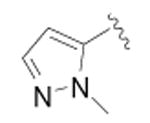
|
CH3 | 89 ± 35 | 3 |
| 19 |
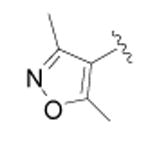
|
CH3 | 2133 ± 226 | 3 |
| 20 | N-Me-piperazinyl | CH3 | 840 ± 132 | 3 |
| 21 | morpholino | CH3 | 51 ± 7 | 3 |
| 22 | phenyl | MeOCH2CH2O- | >10,000 | 3 |
| 23 | phenyl | Me2N- | 699 ± 87 | 3 |
| 24 | phenyl | N-Me-piperazinyl | 1183 ± 206 | 5 |
antiprion potency in ScN2a-cl3 cells by ELISA; SEM is calculated as standard deviation divided by the square root of n.
number of repetitions.
