Table 3.
Antiprion potency for AMT analogs with amide C-groups.
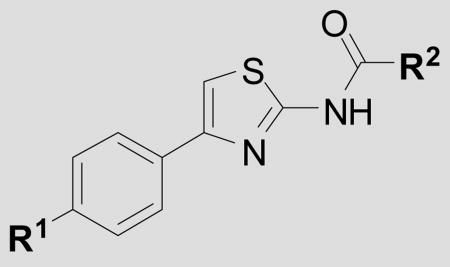
| ||||
|---|---|---|---|---|
| Cmpd | R1 = | R2 = | EC50 ± SEM(nM)[a] | n[B] |
| 34 | phenyl | cyclopropyl | 248 ± 67 | 4 |
| 35 | phenyl | CH3 | 119 ± 10 | 3 |
| 36 | phenyl | MeOCH2- | 1588 ± 295 | 3 |
| 37 | phenyl | i-Pr | >10,000 | 3 |
| 38 | phenyl | phenyl | >10,000 | 3 |
| 39 | pyridin-4-yl | cyclopropyl | 173 ± 16 | 3 |
| 40 | 2-Me-pyridin-3-yl | cyclopropyl | >10,000 | 3 |
| 41 | 4-Me2N-pyridin-3-yl | cyclopropyl | 109 ± 12 | 3 |
| 42 |
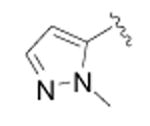
|
cyclopropyl | >10,000 | 3 |
| 43 |
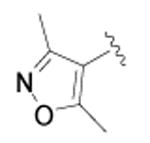
|
cyclopropyl | >10,000 | 3 |
| 44 |

|
cyclopropyl | 1315 ± 233 | 4 |
| 45 |
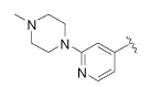
|
cyclopropyl | 682 ± 100 | 3 |
| 46 |
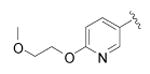
|
cyclopropyl | 118 ± 11 | 3 |
| 47 |
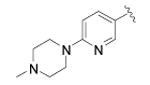
|
cyclopropyl | 70 ± 23 | 4 |
antiprion potency in ScN2a-cl3 cells by ELISA; SEM is calculated as standard deviation divided by the square root of n.
number of repetitions.
