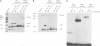Abstract
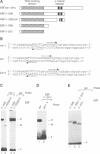
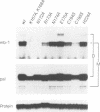
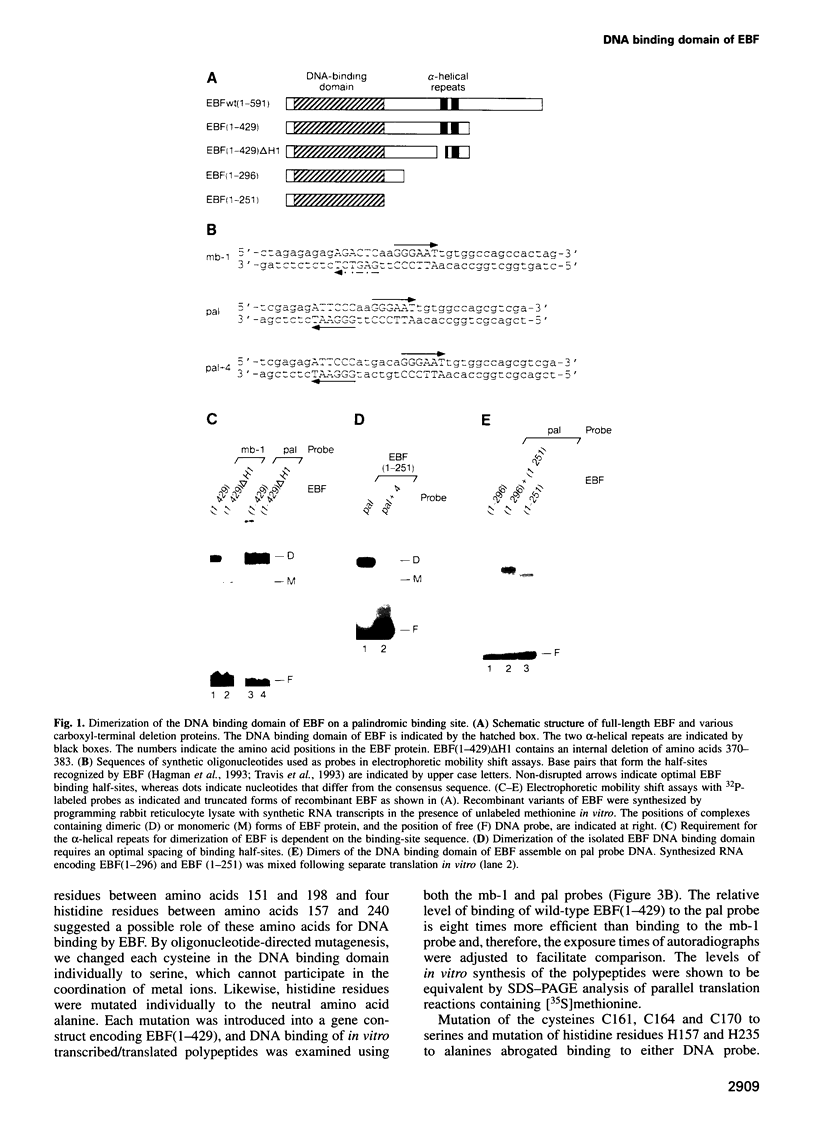
Early B cell factor (EBF) was identified and cloned as a transcription factor expressed specifically in B lymphocytes and adipocytes. This protein was also identified as olfactory factor 1 (Olf-1) in olfactory neurons. In this study, we analyzed the structural requirements for DNA binding, homodimerization and transcriptional activation by EBF. A carboxyl-terminal region, containing a repeat of alpha-helices related to the helix-loop-helix motif, is important for dimerization of EBF in solution and can confer dimerization upon a heterologous DNA binding protein. The amino-terminal DNA binding domain by itself is monomeric, but can mediate assembly of dimers on optimized and correctly spaced half-sites. Mutational analysis of the DNA binding domain of EBF indicated that a novel zinc coordination motif consisting of H-X3-C-X2-C-X5-C is important for DNA recognition. Deletion analysis and transfer of regions of EBF onto a heterologous DNA binding domain identified a serine/threonine-rich transcriptional activation domain. Moreover, the DNA binding domain of EBF can mediate transcriptional activation from optimized binding sites. Thus, EBF contains both a complex DNA binding domain that allows for dimerization and transcriptional activation, and additional dimerization and activation domains.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baldarelli R. M., Mahoney P. A., Salas F., Gustavson E., Boyer P. D., Chang M. F., Roark M., Lengyel J. A. Transcripts of the Drosophila blastoderm-specific locus, terminus, are concentrated posteriorly and encode a potential DNA-binding finger. Dev Biol. 1988 Jan;125(1):85–95. doi: 10.1016/0012-1606(88)90061-9. [DOI] [PubMed] [Google Scholar]
- Berg J. M. Zinc fingers and other metal-binding domains. Elements for interactions between macromolecules. J Biol Chem. 1990 Apr 25;265(12):6513–6516. [PubMed] [Google Scholar]
- Campbell K. S., Cambier J. C. B lymphocyte antigen receptors (mIg) are non-covalently associated with a disulfide linked, inducibly phosphorylated glycoprotein complex. EMBO J. 1990 Feb;9(2):441–448. doi: 10.1002/j.1460-2075.1990.tb08129.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman J. E. Zinc proteins: enzymes, storage proteins, transcription factors, and replication proteins. Annu Rev Biochem. 1992;61:897–946. doi: 10.1146/annurev.bi.61.070192.004341. [DOI] [PubMed] [Google Scholar]
- Culp J. S., Webster L. C., Friedman D. J., Smith C. L., Huang W. J., Wu F. Y., Rosenberg M., Ricciardi R. P. The 289-amino acid E1A protein of adenovirus binds zinc in a region that is important for trans-activation. Proc Natl Acad Sci U S A. 1988 Sep;85(17):6450–6454. doi: 10.1073/pnas.85.17.6450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danielsen M., Hinck L., Ringold G. M. Two amino acids within the knuckle of the first zinc finger specify DNA response element activation by the glucocorticoid receptor. Cell. 1989 Jun 30;57(7):1131–1138. doi: 10.1016/0092-8674(89)90050-0. [DOI] [PubMed] [Google Scholar]
- Diffley J. F., Stillman B. Similarity between the transcriptional silencer binding proteins ABF1 and RAP1. Science. 1989 Nov 24;246(4933):1034–1038. doi: 10.1126/science.2511628. [DOI] [PubMed] [Google Scholar]
- Fawell S. E., Lees J. A., White R., Parker M. G. Characterization and colocalization of steroid binding and dimerization activities in the mouse estrogen receptor. Cell. 1990 Mar 23;60(6):953–962. doi: 10.1016/0092-8674(90)90343-d. [DOI] [PubMed] [Google Scholar]
- Feldhaus A. L., Mbangkollo D., Arvin K. L., Klug C. A., Singh H. BLyF, a novel cell-type- and stage-specific regulator of the B-lymphocyte gene mb-1. Mol Cell Biol. 1992 Mar;12(3):1126–1133. doi: 10.1128/mcb.12.3.1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freedman L. P., Yamamoto K. R., Luisi B. F., Sigler P. B. More fingers in hand. Cell. 1988 Aug 12;54(4):444–444. doi: 10.1016/0092-8674(88)90063-3. [DOI] [PubMed] [Google Scholar]
- Giniger E., Ptashne M. Transcription in yeast activated by a putative amphipathic alpha helix linked to a DNA binding unit. Nature. 1987 Dec 17;330(6149):670–672. doi: 10.1038/330670a0. [DOI] [PubMed] [Google Scholar]
- Green S., Kumar V., Theulaz I., Wahli W., Chambon P. The N-terminal DNA-binding 'zinc finger' of the oestrogen and glucocorticoid receptors determines target gene specificity. EMBO J. 1988 Oct;7(10):3037–3044. doi: 10.1002/j.1460-2075.1988.tb03168.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagman J., Belanger C., Travis A., Turck C. W., Grosschedl R. Cloning and functional characterization of early B-cell factor, a regulator of lymphocyte-specific gene expression. Genes Dev. 1993 May;7(5):760–773. doi: 10.1101/gad.7.5.760. [DOI] [PubMed] [Google Scholar]
- Hagman J., Grosschedl R. An inhibitory carboxyl-terminal domain in Ets-1 and Ets-2 mediates differential binding of ETS family factors to promoter sequences of the mb-1 gene. Proc Natl Acad Sci U S A. 1992 Oct 1;89(19):8889–8893. doi: 10.1073/pnas.89.19.8889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagman J., Travis A., Grosschedl R. A novel lineage-specific nuclear factor regulates mb-1 gene transcription at the early stages of B cell differentiation. EMBO J. 1991 Nov;10(11):3409–3417. doi: 10.1002/j.1460-2075.1991.tb04905.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollenberg S. M., Evans R. M. Multiple and cooperative trans-activation domains of the human glucocorticoid receptor. Cell. 1988 Dec 2;55(5):899–906. doi: 10.1016/0092-8674(88)90145-6. [DOI] [PubMed] [Google Scholar]
- Hombach J., Leclercq L., Radbruch A., Rajewsky K., Reth M. A novel 34-kd protein co-isolated with the IgM molecule in surface IgM-expressing cells. EMBO J. 1988 Nov;7(11):3451–3456. doi: 10.1002/j.1460-2075.1988.tb03219.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hombach J., Tsubata T., Leclercq L., Stappert H., Reth M. Molecular components of the B-cell antigen receptor complex of the IgM class. Nature. 1990 Feb 22;343(6260):760–762. doi: 10.1038/343760a0. [DOI] [PubMed] [Google Scholar]
- Kozak M. At least six nucleotides preceding the AUG initiator codon enhance translation in mammalian cells. J Mol Biol. 1987 Aug 20;196(4):947–950. doi: 10.1016/0022-2836(87)90418-9. [DOI] [PubMed] [Google Scholar]
- Kudrycki K., Stein-Izsak C., Behn C., Grillo M., Akeson R., Margolis F. L. Olf-1-binding site: characterization of an olfactory neuron-specific promoter motif. Mol Cell Biol. 1993 May;13(5):3002–3014. doi: 10.1128/mcb.13.5.3002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunkel T. A. Rapid and efficient site-specific mutagenesis without phenotypic selection. Proc Natl Acad Sci U S A. 1985 Jan;82(2):488–492. doi: 10.1073/pnas.82.2.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurokawa R., Yu V. C., När A., Kyakumoto S., Han Z., Silverman S., Rosenfeld M. G., Glass C. K. Differential orientations of the DNA-binding domain and carboxy-terminal dimerization interface regulate binding site selection by nuclear receptor heterodimers. Genes Dev. 1993 Jul;7(7B):1423–1435. doi: 10.1101/gad.7.7b.1423. [DOI] [PubMed] [Google Scholar]
- Lees J. A., Fawell S. E., Parker M. G. Identification of two transactivation domains in the mouse oestrogen receptor. Nucleic Acids Res. 1989 Jul 25;17(14):5477–5488. doi: 10.1093/nar/17.14.5477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lees J. A., Fawell S. E., White R., Parker M. G. A 22-amino-acid peptide restores DNA-binding activity to dimerization-defective mutants of the estrogen receptor. Mol Cell Biol. 1990 Oct;10(10):5529–5531. doi: 10.1128/mcb.10.10.5529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma J., Ptashne M. A new class of yeast transcriptional activators. Cell. 1987 Oct 9;51(1):113–119. doi: 10.1016/0092-8674(87)90015-8. [DOI] [PubMed] [Google Scholar]
- Mader S., Kumar V., de Verneuil H., Chambon P. Three amino acids of the oestrogen receptor are essential to its ability to distinguish an oestrogen from a glucocorticoid-responsive element. Nature. 1989 Mar 16;338(6212):271–274. doi: 10.1038/338271a0. [DOI] [PubMed] [Google Scholar]
- Matsuuchi L., Gold M. R., Travis A., Grosschedl R., DeFranco A. L., Kelly R. B. The membrane IgM-associated proteins MB-1 and Ig-beta are sufficient to promote surface expression of a partially functional B-cell antigen receptor in a nonlymphoid cell line. Proc Natl Acad Sci U S A. 1992 Apr 15;89(8):3404–3408. doi: 10.1073/pnas.89.8.3404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merino E., Osuna J., Bolívar F., Soberón X. A general, PCR-based method for single or combinatorial oligonucleotide-directed mutagenesis on pUC/M13 vectors. Biotechniques. 1992 Apr;12(4):508–510. [PubMed] [Google Scholar]
- Miller J., McLachlan A. D., Klug A. Repetitive zinc-binding domains in the protein transcription factor IIIA from Xenopus oocytes. EMBO J. 1985 Jun;4(6):1609–1614. doi: 10.1002/j.1460-2075.1985.tb03825.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagai K., Thøgersen H. C. Synthesis and sequence-specific proteolysis of hybrid proteins produced in Escherichia coli. Methods Enzymol. 1987;153:461–481. doi: 10.1016/0076-6879(87)53072-5. [DOI] [PubMed] [Google Scholar]
- Pabo C. O., Sauer R. T. Transcription factors: structural families and principles of DNA recognition. Annu Rev Biochem. 1992;61:1053–1095. doi: 10.1146/annurev.bi.61.070192.005201. [DOI] [PubMed] [Google Scholar]
- Perlmann T., Rangarajan P. N., Umesono K., Evans R. M. Determinants for selective RAR and TR recognition of direct repeat HREs. Genes Dev. 1993 Jul;7(7B):1411–1422. doi: 10.1101/gad.7.7b.1411. [DOI] [PubMed] [Google Scholar]
- Pfahl M. Vertebrate receptors: molecular biology, dimerization and response elements. Semin Cell Biol. 1994 Apr;5(2):95–103. doi: 10.1006/scel.1994.1013. [DOI] [PubMed] [Google Scholar]
- Strum K. Acid connections. Curr Biol. 1991 Jun;1(3):188–191. doi: 10.1016/0960-9822(91)90230-t. [DOI] [PubMed] [Google Scholar]
- Travis A., Hagman J., Grosschedl R. Heterogeneously initiated transcription from the pre-B- and B-cell-specific mb-1 promoter: analysis of the requirement for upstream factor-binding sites and initiation site sequences. Mol Cell Biol. 1991 Nov;11(11):5756–5766. doi: 10.1128/mcb.11.11.5756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Travis A., Hagman J., Hwang L., Grosschedl R. Purification of early-B-cell factor and characterization of its DNA-binding specificity. Mol Cell Biol. 1993 Jun;13(6):3392–3400. doi: 10.1128/mcb.13.6.3392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai S. Y., Carlstedt-Duke J., Weigel N. L., Dahlman K., Gustafsson J. A., Tsai M. J., O'Malley B. W. Molecular interactions of steroid hormone receptor with its enhancer element: evidence for receptor dimer formation. Cell. 1988 Oct 21;55(2):361–369. doi: 10.1016/0092-8674(88)90059-1. [DOI] [PubMed] [Google Scholar]
- Umesono K., Evans R. M. Determinants of target gene specificity for steroid/thyroid hormone receptors. Cell. 1989 Jun 30;57(7):1139–1146. doi: 10.1016/0092-8674(89)90051-2. [DOI] [PubMed] [Google Scholar]
- Venkitaraman A. R., Williams G. T., Dariavach P., Neuberger M. S. The B-cell antigen receptor of the five immunoglobulin classes. Nature. 1991 Aug 29;352(6338):777–781. doi: 10.1038/352777a0. [DOI] [PubMed] [Google Scholar]
- Wang M. M., Reed R. R. Molecular cloning of the olfactory neuronal transcription factor Olf-1 by genetic selection in yeast. Nature. 1993 Jul 8;364(6433):121–126. doi: 10.1038/364121a0. [DOI] [PubMed] [Google Scholar]
- Wang M. M., Tsai R. Y., Schrader K. A., Reed R. R. Genes encoding components of the olfactory signal transduction cascade contain a DNA binding site that may direct neuronal expression. Mol Cell Biol. 1993 Sep;13(9):5805–5813. doi: 10.1128/mcb.13.9.5805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westin G., Gerster T., Müller M. M., Schaffner G., Schaffner W. OVEC, a versatile system to study transcription in mammalian cells and cell-free extracts. Nucleic Acids Res. 1987 Sep 11;15(17):6787–6798. doi: 10.1093/nar/15.17.6787. [DOI] [PMC free article] [PubMed] [Google Scholar]