Abstract
Along with the increasing need for living-donor liver transplantation (LDLT), the issue of organ shortage has become a serious problem. Therefore, the use of organs from elderly donors has been increasing. While the short-term results of LDLT have greatly improved, problems affecting the long-term outcome of transplant patients remain unsolved. Furthermore, since contradictory data have been reported with regard to the relationship between donor age and LT/LDLT outcome, the question of whether the use of elderly donors influences the long-term outcome of a graft after LT/LDLT remains unsettled. To address whether hepatocyte telomere length reflects the outcome of LDLT, we analyzed the telomere lengths of hepatocytes in informative biopsy samples from 12 paired donors and recipients (grafts) of pediatric LDLT more than 5 years after adult-to-child LDLT because of primary biliary atresia, using quantitative fluorescence in situ hybridization (Q-FISH). The telomere lengths in the paired samples showed a robust relationship between the donor and grafted hepatocytes (r = 0.765, p = 0.0038), demonstrating the feasibility of our Q-FISH method for cell-specific evaluation. While 8 pairs showed no significant difference between the telomere lengths for the donor and the recipient, the other 4 pairs showed significantly shorter telomeres in the recipient than in the donor. Multiple regression analysis revealed that the donors in the latter group were older than those in the former (p = 0.001). Despite the small number of subjects, this pilot study indicates that donor age is a crucial factor affecting telomere length sustainability in hepatocytes after pediatric LDLT, and that the telomeres in grafted livers may be elongated somewhat longer when the grafts are immunologically well controlled.
Introduction
Liver transplantation (LT) has been carried out worldwide for patients with end-stage liver failure [1]. Since the supply of cadaveric liver grafts is far short of the number of patients awaiting transplantation, living-donor liver transplantation (LDLT) has emerged as a critical surgical option for the patients. Along with the increasing need for LDLT, the issue of organ shortage continues to extend worldwide and has become a serious problem. Therefore, the use of organs from marginal donors, such as the elderly, has been increasing [2]. The short-term results of LDLT have greatly improved because of advances in surgical techniques, antiviral therapy and, particularly, immunosuppressive regimens [3], [4], [5], [6]. However, new problems, including recurrence of hepatitis C or hepatocellular carcinoma and development of de novo malignancies, have arisen as major problems affecting the long-term outcome of transplant patients [7]. Ensuring that a transplanted organ has a potentially sufficient working life remains one of the most important issues associated with LDLT [8], [9], [10]. In this context, a fundamental question has arisen concerning the senescence (aging process) of organs that are transplanted from elderly donors into young recipients. LDLT provides a unique and valuable means of answering this question, because it allows us to compare the characteristics of hepatocyte samples from an originally identical liver, part of which continues to be viable and functional in the donor, and another part that has functioned in a young recipient for a number of years after transplantation.
Human telomeric DNA is considered to protect chromosomes against degeneration, fusion, and loss [11]. In many human organs and tissues, including the liver [12], [13], in vivo telomere shortening occurs with aging, and is accelerated by various environmental factors such as oxidative stress, chronic inflammation, social stress, alcohol, and transplantation [14], [15], [16], [17], [18], [19], [20]. The telomere hypothesis of cellular aging suggests that when telomere shortening reaches a critical level, a DNA damage checkpoint mechanism may be initiated and the cells stop dividing [21], [22]. Therefore, measurement of telomere length is expected to provide information for evaluating the life span of a cell.
Since Lansdorp et al. devised the quantitative fluorescence in situ hybridization (Q-FISH) method [23], several studies of telomere length using various Q-FISH methods have been reported by different groups including us [16], [24], [25], [26], [18]. However there have been no reports of telomere length measurement in liver grafts after LDLT. We had an opportunity to study 12 pairs of samples obtained at the same time by needle biopsy from the donors and recipients more than after 5 years after LDLT. To address the question of whether telomere shortening is accelerated in the graft relative to the native liver remaining in the donor after pediatric LDLT, we used Q-FISH to measure the telomere length in hepatocytes.
Materials and Methods
Patients
We offered all of the LDLT patients (donors and recipients) being treated at our institution a chance to participate in this pilot study, and twelve pairs volunteered to take part. These twelve cases were considered suitable for our study protocol, which involved examination of a pair of liver biopsy specimens from both the donor and recipient after a follow-up period of more than 5 years after LDLT. No other selection criteria were adopted. These informative cases were obtained from among 134 recipients who had undergone LDLT at the Jichi Medical University Hospital between May 2001 and March 2009. The overall patient survival rate in our department was 95.0% (134/141). The ages, relationships (parent to child in all pairs) and post-LT survivals of the 12 pairs of donors and recipients are listed in Table 1. All of the patients examined in this study had end-stage biliary atresia, a condition that accounted for 73% of total LDLT operations performed at our hospital. Hepatocellular reserve capacity after Kasai portoenterostomy is usually evaluated in terms of the pediatric end-stage liver disease (PELD) score [27], [28]. The PELD score is calculated on the basis of several objective values, the main components being age, growth failure (based on sex, height, and weight), albumin (g/dl), prothrombin time (international normalized ratio), and total bilirubin (mg/dl) (shown in Table S2).
Table 1. Characteristics of donors and patients.
| Group | Pair No. | Age at LDLT | Relation | Biopsy Duration from LDLT | Blood type | HLA mismatch number | Median value of NTCR | p value | |
| Lower NTCR Group | Donor | 36y8m | Father | A+ | 1.51 | ||||
| 1 | 12y6m | 3 | p<0.001 | ||||||
| Recipient | 1y3m | Daughter | A+ | 1.07 | |||||
| Donor | 44y10m | Father | O+ | 0.42 | |||||
| 2 | 11y4m | 2 | p<0.001 | ||||||
| Recipient | 8y4m | Son | O+ | 0.27 | |||||
| Donor | 39y0m | Father | A+ | 1.04 | |||||
| 3 | 5y6m | 3 | p = 0.015 | ||||||
| Recipient | 1y10m | Daughter | A+ | 0.78 | |||||
| Donor | 42y3m | Mother | B+ | 1.13 | |||||
| 4 | 5y6m | 3 | p<0.001 | ||||||
| Recipient | 9m | Daughter | B+ | 0.73 | |||||
| Comparable NTCR Group | Donor | 36y7m | Father | O+ | 1.05 | ||||
| 5 | 16y10m | 3 | p = 0.265 | ||||||
| Recipient | 9m | Daughter | O+ | 0.95 | |||||
| Donor | 35y7m | Father | B+ | 0.57 | |||||
| 6 | 15y3m | 3 | p = 0.948 | ||||||
| Recipient | 1y7m | Daughter | AB+ | 0.53 | |||||
| Donor | 38y4m | Father | O+ | 0.99 | |||||
| 7 | 5y4m | 3 | p = 0.366 | ||||||
| Recipient | 7y3m | Daughter | O+ | 0.96 | |||||
| Donor | 37y9m | Stepfather | B+ | 0.37 | |||||
| 8 | 13y0m | 6 | p = 0.065 | ||||||
| Recipient | 4y1m | Son | B+ | 0.46 | |||||
| Donor | 28y7m | Mother | O+ | 0.56 | |||||
| 9 | 10y6m | 2 | p = 0.109 | ||||||
| Recipient | 1y0m | Son | A+ | 0.77 | |||||
| Donor | 32y1m | Father | A+ | 1.03 | |||||
| 10 | 8y9m | 3 | p = 0.056 | ||||||
| Recipient | 2y6m | Daughter | A+ | 1.2 | |||||
| Donor | 34y8m | Father | B+ | 0.88 | |||||
| 11 | 6y7m | 3 | p = 0.115 | ||||||
| Recipient | 2y7m | Daughter | B+ | 1.02 | |||||
| Donor | 29y5m | Father | A+ | 0.77 | |||||
| 12 | 5y5m | 2 | p = 0.342 | ||||||
| Recipient | 1y5m | Daughter | A+ | 0.82 |
NTCR: normalized telomere centromere ratio.
Lower NTCR group: Median NTCRs of the recipient hepatocyte were significantly lower than those of the donor.
Comparable NTCR group: Median NTCRs of the recipient hepatocyte were neither significantly lower nor higher than those of the donor.
Median donor age and median recipient age at the time of LDLT were 36.6 y (28.6 y to 44.8 y) and 1.7 y (0.8 y to 8.3 y), respectively.
Median donor age and median recipient age at the time of biopsy were 46.2 y (34.8 y to 56.2 y) and 12.0 y (6.3 y to 19.7 y), respectively.
The median interval from LDLT to biopsy was 9.6 y.
The ethics committees of Jichi Medical University and Tokyo Metropolitan Institute of Gerontology approved the use of liver biopsy for this study. The participants provided written informed consent before each liver biopsy in accordance with the Declaration of Helsinki. Parental written permission was obtained when the patients were younger than 20 years old.
Surgical procedure and postoperative management
The surgical procedure (left side liver graft) and postoperative management (immunosuppression started using tacrolimus and steroid) for LDLT were basically the same as described previously [29]. The immunosuppression status of the recipients at the time of biopsy is summarized in Table 2, and the most recent status is summarized in Table S3. All of the grafted livers from donors were confirmed to be histologically normal by biopsy at the time of LDLT.
Table 2. Clinical and pathological status of donors and recipients.
| Group | Pair No. | Major complication of recipient | Pathological findings | Immunosuppression status | |
| Lower NTCR Group | Donor | (-) | |||
| 1 | Recipient | Chronic hepatitis | Mild interface hepatitis | Tacrolimus: twice a day | |
| de novo HBV | Micophenolate mofetil | ||||
| Donor | (-) | ||||
| 2 | Recipient | Mild interface hepatitis | Tacrolimus: twice a day | ||
| Micophenolate mofetil | |||||
| Steroid | |||||
| Donor | (-) | ||||
| 3 | Recipient | Tacrolimus: twice a day | |||
| HV stenosis | Marked bridging fibrosis | Micophenolate mofetil | |||
| Steroid | |||||
| Donor | (-) | ||||
| 4 | Recipient | Unstable liver function | Mild lobular hepatitis | Cyclosporine: twice a day | |
| Micophenolate mofetil | |||||
| Comparable NTCR Group | Donor | Moderate steatosis | |||
| 5 | Recipient | de novo HBV | (-) | Cyclosporine: twice a day | |
| (seroconversion) | Micophenolate mofetil | ||||
| Donor | |||||
| 6 | Recipient | Liver dysfunction | Marked bridging fibrosis | Tacrolimus: twice a day | |
| HV stenosis | Micophenolate mofetil | ||||
| Steroid | |||||
| 7 | Donor | (-) | |||
| Recipient | None | Mild interface hepatitis | Tacrolimus: twice a day | ||
| Donor | Moderate steatosis | ||||
| 8 | (NASH suspected) | ||||
| Recipient | Biliary stricture | Canalicular cholestasis | Cyclosporine: twice a day | ||
| Donor | (-) | ||||
| 9 | Recipient | HV stenosis | Mild interface hepatitis | Cyclosporine: twice a day | |
| Micophenolate mofetil | |||||
| 10 | Donor | (-) | |||
| Recipient | None | (-) | Complete cessation | ||
| 11 | Donor | (-) | |||
| Recipient | None | (-) | Tacrolimus: once a week | ||
| Donor | Moderate steatosis | ||||
| 12 | Recipient | Unstable liver function | (-) | Tacrolimus: twice a day | |
| Micophenolate mofetil |
HBV: hepatitis B virus, HV: hepatic vein, NTCR: normalized telomere centromere ratio, NASH: none-alcoholic steatotic hepatitis, (-): almost normal.
Lower NTCR group: Median NTCRs of the recipient hepatocyte was significantly lower than those of the donor.
Compatible NTCR group: Median NTCRs of the recipient hepatocyte was not significantly lower nor higher than those of the donor.
Episodes of acute cellular rejection (ACR) are summarized in Table S3. Liver biopsy was indicated when any liver function parameters (aspartate aminotransferase, alanine aminotransferase, gamma-glutamyl transpeptidase, and total bilirubin) showed abnormal values suggesting ACR. All episodes of ACR were diagnosed on the basis of liver biopsy pathology. Highly experienced pathologists evaluated the degree of portal infiltration by lymphocytes, bile duct inflammation or damage, and venous endothelial inflammation, according to the Banff scheme.
Preparation of biopsy specimens and histological assessment
The liver specimens were obtained by ultrasonography-guided core needle (16 gauge) biopsy from the donor and recipient under local anesthesia. These paired biopsies were performed within a week of each other. The donors fully approved biopsy of their liver because they expressed a desire to know the pathological state of the liver and the pathological relationship between the donor and recipient livers after receiving a full explanation about all the potential complications of the biopsy.
The liver biopsy specimens were fixed in 10% buffered formalin solution and embedded in paraffin. The specimens were then sliced into sections 3 μm thick for hematoxylin and eosin (HE) staining and into sections 2 μm thick for Q-FISH. All sections included more than 6 portal tracts. Histological findings were assessed by a histopathologist who was an expert in liver pathology (K.M), and described based on the criteria proposed by the Banff Working Group [30].
Telomere length quantification by Q-FISH
The slides were processed with the Q-FISH method, as reported previously [18], [26]. In brief, the telomeres were labeled with Cy3-labeled CCCTAACCCTAACCCTAA peptide nucleic acid probe (teloC: F1002; Fasmac, Japan) and the centromeres were labeled with a FITC-labeled CTTCGTTGGAAACGGGGT peptide nucleic acid probe (CENP1: a non-specific centromere probe) (custom-made, Fasmac). The chromosome preparations were counterstained with 40, 6-diamino-2-phenylindole (DAPI, Molecular Probes, Eugene, OR, USA). Microscopical images were captured with the Image-Pro Plus software package (version 5.0, Media Cybernetics Co. Ltd., Silver Spring, MD, USA), and analyzed using our own telomere analysis software ‘TissueTelo Ver. 2’. The telomere length estimate for each nucleus was defined as the ratio of the detected signal intensity of the telomere relative to that of the centromere (telomere-to-centromere ratio: TCR). At least 127 cells (median: 208 cells, range: 127–463 cells) were analyzed in each case.
TCR normalization using a cell block
As a control for variations in sample preparation, we also performed Q-FISH on a cell block-section of a cultured fibroblast strain, TIG-1 [31] (34 population doubling levels, terminal restriction fragment length determined as 8.6 kilo base pairs), placed on the same slide as each hepatic section. Every TCR for hepatocytes was divided by the median TCR for the cell block on the same slide to give the normalized TCR (NTCR) [26].
Limitation of the study
The main limitation of this study was the small number of subjects for whom NTCR values for the paired donor and graft could be determined. However, despite the limited number (12 pairs) of informative cases, the major findings were statistically significant.
Statistical analyses
The values of measured variables were expressed as mean ± standard deviation or median with a range of values. The median NTCRs of the donor and recipient hepatocytes were compared between cases using the Mann-Whitney test or Wilcoxon test. Correlations were analyzed with the Spearman correlation coefficient test and a single regression analysis using the software package Dr.SPSS II (SPSS, Chicago, IL). The relationships among NTCR values, donor age and group (lower versus comparable NTCR) were assessed by multiple regression analyses using StatView software version 5.0 (SAS Institute Inc.). Differences were considered significant at p<0.05.
Results
Quantification of telomere length in liver tissue of donors and recipients using Q-FISH
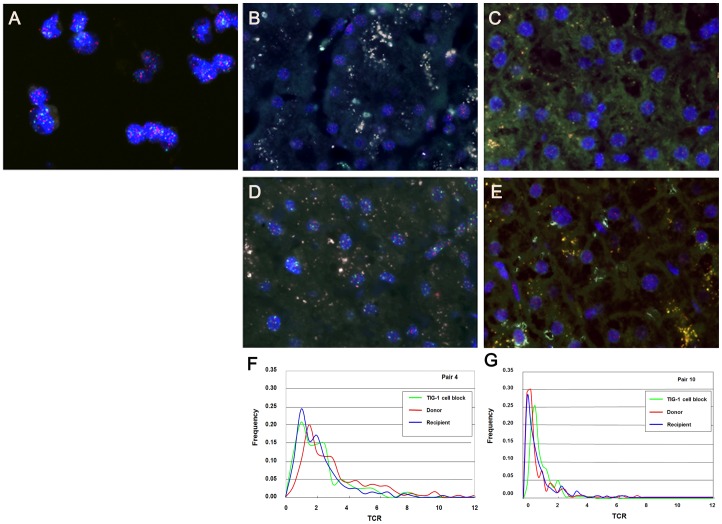
Figure 1A shows a Q-FISH image of a TIG-1 cell block placed on the same slide as liver sections from the donor and recipient. Representative Q-FISH images of liver tissues from the donor and recipient are shown in Figure 1B, D (pair 4), and Figure 1C, E (pair 10), in which the telomere signals (Cy3) appear red while the centromere signals (FITC) appear green on the DAPI-stained nuclei. With reference to HE-stained serial sections (Shown in Figure 2), we were able to distinguish hepatocytes from other cell types (including ductal cells, interstitial fibroblasts and infiltrating lymphocytes). The profiles of the measured telomere:centromere ratio (TCR) are plotted in Figure 1F, G. Since the frequency of TCR values did not show a normal distribution, we chose the median values as representative. The median values of the TCR for the donor and recipient hepatocytes are divided by the TCR value for TIG-1 cells and obtained normalized TCR (NTCR) (summarized in Table 1). Among interstitial cells, strong signal intensity for Cy3 was often evident in fibroblasts.
Figure 1. Representative Q-FISH images of TIG-1 and paired liver tissues (red: Cy3, telomere signals; green: FITC, centromere signals; blue: DAPI, nuclei).
(A) Q-FISH image reveals the TIG-1 cell block placed on the same slide together with liver sections. Telomere (Cy3, red) and centromere (FITC, green) signals are evident (original magnification ×400). (B) Q-FISH images of pair 4 donor. Telomere and centromere signals are evident in the nuclei (original magnification ×400). (C) Q-FISH images of pair 10 donor. (original magnification ×400). (D) Q-FISH image of pair 4 recipient in the lower NTCR group reveals weaker telomere signals (red) than those in the paired donor (Figure 1B) (original magnification ×400). (E) Q-FISH images of pair 10 recipient, showing brighter telomere signals (red) than those in the paired donor (Figure 2D) (original magnification ×400). (F) Distributions of the telomere intensity given by telomere-to-centromere ratio (TCR) in TIG-1 and hepatocytes from paired liver tissues of pair 4 samples. Green: TIG-1 cells in a cell block, red: hepatocytes in donor, blue: hepatocytes in recipient grafted liver. (G) TCR distribution in TIG-1, and hepatocytes from paired liver tissues of pair 10 samples.
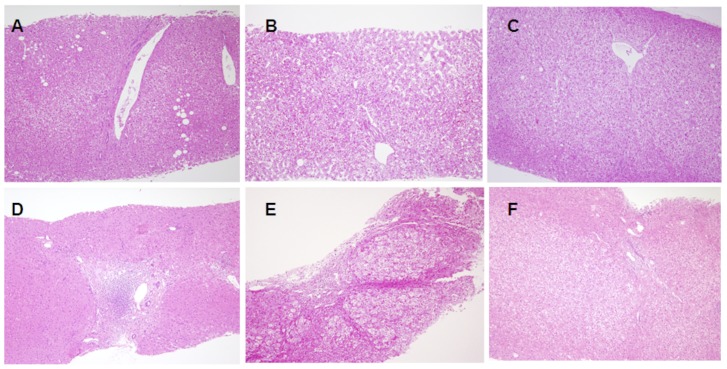
Figure 2. Representative histological and histopathological features of the livers.
(A) Histological findings in pair 1 donor, showing almost normal feature (HE, original magnification ×100). (B) Histological findings in pair 3 donor, showing almost normal feature (HE, original magnification ×100). (C) Histological findings in pair 10 donor, showing almost normal feature (HE, original magnification ×100). (D) Histological findings in pair 1 recipient in the lower NTCR group, showing mild interface hepatitis due to infection with hepatitis B virus via the graft from the HBV carrier donor (HE, original magnification ×40). (E) Histological findings in pair 3 recipient in the lower NTCR group, showing marked bridging fibrosis due to repeated hepatic vein stenosis (HE, original magnification ×40). (F) Histological findings in pair 10 recipient, with good liver function and complete withdrawal of immunosuppressant, showing almost normal feature (HE, original magnification ×100).
Relationship between NTCR values for the donor and recipient
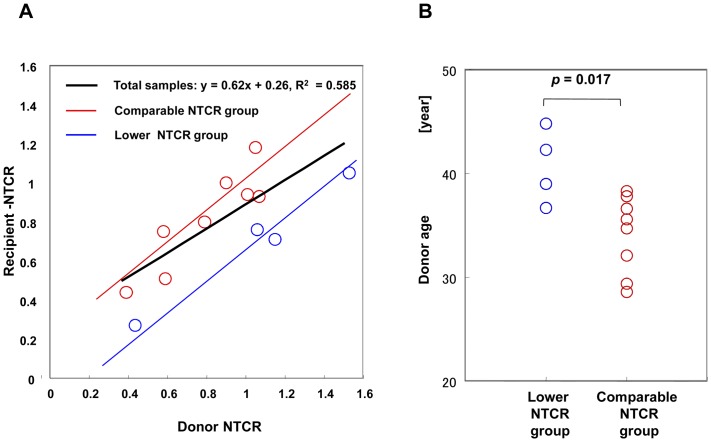
Scatter plot analysis of the median NTCR values for hepatocytes provided a robust correlation between donors and recipients (p = 0.0038) (Figure 3A). The slope provided by the regression analysis was less than 1, and the mean of the median NTCR value for hepatocytes from donors and that of hepatocytes from recipients (grafts) was 0.82 and 0.78, respectively. Hence, the NTCR values for recipients generally tended to be lower than those for the donors. However, in individual cases, even if the NTCR value for the grafted liver was higher or lower than that for the donor liver, the difference was not statistically significant (p = 0.092).
Figure 3.
(A) Scatter plot analysis of the relationship between NTCRs in donor hepatocytes and those in recipient hepatocytes. A regression line was obtained from all of the paired subjects (n = 12), y (recipient NTCR) = 0.62x(donor NTCR)+0.26, (r = 0.765, R2 = 0.544, p = 0.0038), shown by a solid black line. By using model 1: Y = β0+β1X1 (age)+β2 X2 (group), that related Y (recipient NTCR) to X1 (donor NTCR) and X2 (group), given that the value for the comparable group was 1 and that for the lower group was 0. This model yielded a regression line from the comparable patient group (n = 8): Y (recipient NTCR) = −0.10+0.79X(donor NTCR) +0.32, shown by a red line; and a regression line from the lower patient group (n = 4): Y (recipient NTCR) = −0.10+0.79X (donor NTCR), shown by a blue line. The difference of recipient NTCR values between the groups was 0.32 and significant (P = 0.001). Red circles: the recipient-donor pair categorized as the comparable NTCR group. Blue circles: the recipient-donor pair categorized as the lower NTCR group. (B) Comparison between donors in the comparable group and those in the lower group by age factor. The difference of donor ages (median values) between the groups was 5 y 6 m and significant (p = 0.017). Red circles, the donors categorized as the comparable NTCR group. Blue circles, the donors categorized as the lower NTCR group.
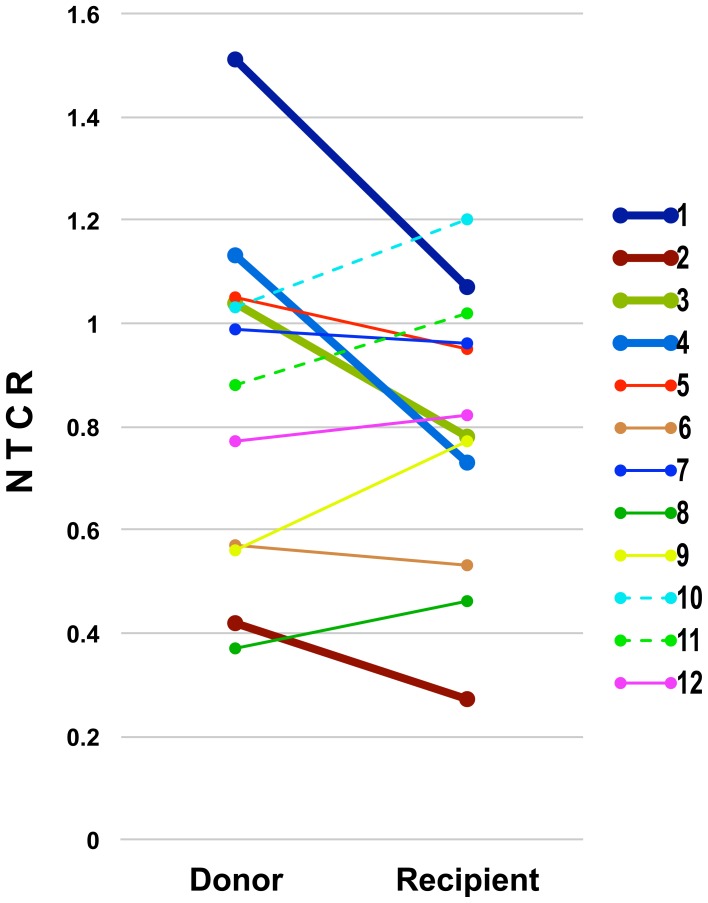
From the relationship between the hepatocyte NTCR values for the donor liver and the graft, we found 4 sample pairs in which the NTCR values for the graft hepatocytes were significantly lower than those for remaining hepatocytes in the donor (Table 1, Figure 4). In the other 8 pairs, although the NTCR values for graft hepatocytes were higher than, comparable to, or lower than the corresponding values for the hepatocytes remaining in the donor, the differences were not statistically significant (Table 1, Figure 4). Hence, we categorized the former as a lower NTCR group and the latter as a comparable NTCR group. Intriguingly, the donors were significantly older in the lower NTCR group (median age 40 y 8 m) than in the corresponding comparable NTCR group (median age 35 y 2 m) (p = 0.017) (Figure 3B). Conversely, the oldest one-third of the donors (pairs 2, 4, 3, 7) demonstrated decreased NTCR values in the recipient, and the youngest one-third of the donors (pairs 9, 12, 10, 11) showed increased NTCR values in the recipient.
Figure 4. Comparison between donor and recipient NTCR values.
Bold lines connect lower NTCR group pairs, and thin lines connect comparable NTCR group pairs. Dashed lines connect the pairs (10 and 11), who achieved complete withdrawal of immunosuppression.
Next, we applied a multiple regression model to assess the difference between the two groups (see Figure 3A legend). This model yielded a regression line indicating that the average ratio of the recipient NTCR to the donor NTCR was 0.794 (p<0.0001), and that the difference between the groups was significant (p = 0.001) (Figure 3A).
With regard to other factors, the ages of the recipients at LDLT showed no significant difference between the two groups (lower NTCR group: median age 1 y 10 m, comparable NTCR group: median age 2 y 1 m, p = 0.799). Also, neither the NTCR values for recipients nor those for the donors differed significantly between the two groups (0.73 vs. 0.89, p = 0.570; 1.04 vs. 0.83, p = 0.241, respectively. The period between LDLT and liver biopsy showed no significant differences between the two groups (lower NTCR group: median duration 8 y 5 m, comparable NTCR group: median period 9 y 8 m, p = 0.734).
Histopathological findings in donors and recipients
The liver biopsy samples from all 4 donors in the lower NTCR group showed normal histological findings. On the other hand, three (pairs 5, 8, and 12) of the 8 donors in the comparable NTCR group showed moderate steatosis.
Among the liver biopsy samples from recipients, significant abnormalities were observed in all of the 4 recipients in the lower NTCR group (mild interface hepatitis in cases 1 and 2, mild lobular hepatitis in case 4, marked bridging fibrosis in case 3) and in 4 of the 8 recipients in the comparable NTCR group (marked bridging fibrosis in case 6, mild interface hepatitis in cases 7 and 9, canalicular cholestasis in case 8) (Figure 2, and summarized in Table 2).
Immunological findings in grafts
In the comparable NTCR group, 3 recipients (pairs 10, 11 and 12) showed a benign course after LDLT, and were treatable with a calcineurin inhibitor at a frequency of less than once a day, or without any immunosuppressant. In particular, the recipient in pair 10, who achieved complete withdrawal of immunosuppression, had a higher median NTCR than the donor, but the difference was of borderline statistical significance (p = 0.056). The recipient in pair 11, who has recently achieved complete withdrawal of immunosuppression, also had a higher median NTCR than the donor. In only one recipient (pair 8) who received a graft from a non-father non-mother (stepfather) donor and was treated with a calcineurin inhibitor, the median NTCR was also higher than that of the donor, but the difference was of borderline statistical significance (p = 0.065). While only one of 8 recipients in the comparable NTCR group (pair 6) continued to require oral maintenance steroid therapy, 2 of 4 recipients (pairs 2 and 3) in the lower NTCR group did so.
Episodes of acute cellular rejection (ACR) were observed in 2 cases in the lower NTCR group and 4 cases in the comparable NTCR group. Among the cases associated with ACR, 4 (pairs 2, 3, 5, 6; cases 2 and 3 being statistically significant) showed decreasing NTCR values in the recipients, and 2 (pairs 8, 12) showed increasing NTCR values in the recipients, but not to a significant degree. With regard to steatosis, 3 of the 4 cases in the comparable NTCR group associated with ACR in the recipient showed steatosis in the donor.
Laboratory data for donors and recipients
The laboratory data for recipients (summarized in Table S1) at liver biopsy have so far revealed no significant differences between the lower NTCR group and the comparable NTCR group. In addition, the PELD scores for recipients (summarized in Table S2) have shown no significant differences between the two groups.
Discussion
In parallel with the increasing need for LDLT, the issue of organ shortage continues to extend worldwide and has become a serious problem. Therefore, the use of organs from marginal donors, such as the elderly, has been increasing [2]. Currently, LDLT is able to ensure survival for more than 5 years, mainly because of improvements in immunosuppression regimens [4], [5], [6]. However, there is still insufficient knowledge of factors that would facilitate long-term or life-long viability or function of the grafted liver after LDLT. Accumulated studies have demonstrated that higher donor age is a risk factor for early relaparotomy and poor outcome [32], [33]; however, some reports have suggested that liver grafts from elderly donors can yield a favorable outcome [34], [35]. Thus, the question of whether the use of grafts from elderly donors may influence the long-term outcome of the graft after LT/LDLT remains unsettled. In addressing possible factors that might have a crucial impact on long-term prognosis, we speculated that hepatocyte telomere length in the recipient might be an indicator of long-term survival potential, as telomere length reportedly reflects not only cellular senescence but also possibly organ aging [36], [37], [13], [38].
The present study represents the first attempt to provide statistical support of telomere length differences in hepatocytes obtained by liver biopsy at almost the same time from both donors and recipients followed up for more than 5 years after pediatric LDLT using the Q-FISH method. The main findings were as follows: Firstly, based on the relationship between the NTCR of the recipient and that of the donor, recipients were classifiable into two groups: a comparable group (showing no significant difference in NTCR) and a lower group (showing a significantly lower NTCR in the recipient than in the donor). However, in 5 of the 8 cases in the comparable group, hepatocyte NTCR values for recipients were larger than those for the donors. Secondly, donors in the lower NTCR group were significantly older than those in the comparable NTCR group, and there was no relationship between the absolute NTCR values per se in the two groups, nor with any of the recipient conditions examined.
With regard to the methodology used for telomere length measurement, numerous approaches have been adopted, including Southern blotting, PCR-based methods, and Q-FISH. Previous studies using Southern blotting have shown that fibrosis or hepatitis can accelerate telomere shortening in the liver [15], [39], [40] in recipients with immune-mediated injury and/or pathological abnormalities of the liver, where the telomere length might be shorter than that of donors [41]. This approach has an underlying problem in that hepatic tissue includes a variety of cell types, such as hepatocytes, macrophages, and fibroblasts, whose telomere lengths are measured simultaneously. On the other hand, Q-FISH makes it possible to analyze the length of hepatocellular telomeres in a cell type-specific manner, although precise calibration is required. We have measured telomere lengths in various human organs and tissues by the Q-FISH method utilizing the centromere signal as an internal control and the terminal restriction fragment length defined fibroblasts as an external control [26], [18], [42]. Recently, we have demonstrated telomere length shortening in the hepatocytes of biliary atresia with severe inflammatory changes and fibrosis using the present Q-FISH method, but failed to reproduce our findings by authentic Southern blotting [43]. The robust correlation between the paired values found in the present study further demonstrated that our Q-FISH method was reliable for cell type-specific analysis.
Our previous population studies demonstrated that telomeres in the liver shorten most rapidly with age among those in the major organs. The telomere shortening in liver is especially rapid in infants, and then the rate of shortening slows from adolescence to middle age; no significant decrease is evident from forties to centenarian age [12], [13], [38]. Hence, from the viewpoint of telomere dynamics, the most critical period for liver aging is assumed to be from birth to forties. Our present data, based on a donor population ranging in age from 28 y 7 m to 44 y 10 m, indicated that donors in the lower NTCR group were significantly older than those in the comparable NTCR group. Taken together, the data strongly suggest that the mechanisms for maintenance of telomere length in the grafts were affected by aging-related changes during early adulthood.
The regeneration of a liver graft in a recipient suggests that the structure and function of the graft might be largely controlled by the host environment [44], organ chimerism, or cell migration from the host to the transplanted liver [45], and that telomerase may play a pivotal role in maintaining telomere length and chromosomal stability in proliferating cells [46]. In relation to LT/LDLT and telomere shortening, several candidate causative factors have been examined, including chronic inflammation, fibrosis, and steatosis. Nakajima et al. have reported that non-alcoholic fatty liver disease with steatosis causes prominent telomere shortening in hepatocytes [47], and therefore steatosis in a grafted liver might potentially accelerate telomere shortening. In the present study, 3 of 12 donors had moderate steatosis (pairs 5, 8, and 12), and all were categorized in the lower NTCR group. Hence, these cases would require careful attention. Liver fibrosis is another histopathological feature of liver disease, and has been reported to be a significant indicator of prognosis after LDLT [48]. In the present study, 2 of the 12 recipients showed marked fibrosis (pairs 3 and 6); both were treated with continuous steroid, but were separated into different groups. Hence, to date, we have been unable to confirm the relationship between fibrosis and telomere length. Hepatitis after LT/LDLT is another critical complication. Three of the lower NTCR group (cases 1, 2, 4) and two of the comparable NTCR group (cases 7, 9) suffered mild hepatitis, and all of them showed a decrease of hepatocyte NTCR in the recipient. These findings are comparable to previous studies [15], [39], [40], [41], and suggest that livers from younger donors may have higher resistance to hepatitis.
With regard to immunological findings, we recently assessed the association of ACR with several factors in 114 LDLTs performed at our institute, including the cases analyzed in the present study. We found that paternal grafts with gender mismatch were associated with a higher incidence of ACR than maternal grafts with gender match, and that there was no significant difference between the donor age groups [49]. Since all of the donors except one in the present study were male, it was not possible to examine any influence of donor gender. However, the latter conclusion is compatible with our present findings. Intriguingly, some graft hepatocytes, including those in pairs 10 and 11, where complete withdrawal of immunosuppression was possible, showed a larger NTCR than those of the donor. Although the difference was not statistically significant, our findings strongly suggest that the telomeres in grafted livers might be elongated somewhat longer when the grafts are immunologically well controlled. Human leukocyte antigen (HLA) compatibility matching is indisputably important in kidney, heart, and bone marrow transplantation, but is not largely considered to have a clinically significant impact in liver transplantation [50], [51]. In the present study, we were unable to demonstrate any significant effect of HLA compatibility on telomere dynamics.
With regard to the relationship between recipient status and LT/LDLT outcome, some studies have demonstrated a correlation between the PELD score before LT and outcome after LT [6], [21]. However, we were unable to find any significant relationship between NTCR dynamics and host status, including PELD score, in this series.
Major arguments concerning the clinical impact of donor age on LT/LDTP outcome have focused mainly on the elderly (60∼65 years of age or more). However, aging phenomena have been observed not only in the elderly but also in young adult or middle-aged individuals [33]. For example, concentrations of growth-hormone/insulin-like growth factor reportedly begin to decline from early adulthood (∼20 years of age), and muscle area/strength from 30 years of age [52], [53]. Our previous population studies of telomere length have demonstrate that telomeres in the skin start to shorten at around 30 years of age [54]; on the other hand, those in the liver shorten rapidly during young age, and continue to do so at 30 to 40 years of age, and then reach a stationary state [12], [13]. The present data provide further evidence for the aging process that occurs during youth and middle age, and shed more light on the importance of the aging process during this earlier period before old age.
Notwithstanding our retrospective study design and the limited number of samples analyzed, our findings lend additional support to the validity of LDLT, because telomere lengths in the majority of the recipients were statistically comparable to those in the native liver of the donor. Furthermore, telomere lengths showed a tendency to be elongated in well-controlled grafted livers. Our data also provide essential clues to the relationship between hepatocyte aging and LDLT outcome, particularly with regard to young/middle-aged individuals. Further studies will undoubtedly reveal other factors that affect hepatocyte telomere length in the grafted liver and their long-term effects on the graft after pediatric LDLT.
Supporting Information
Laboratory data of recipients and donors at biopsy. Lower NTCR group: Median NTCRs of the recipient hepatocyte was significantly lower than those of the donor. Comparable NTCR group: Median NTCRs of the recipient hepatocyte was neither significantly lower nor higher than those of the donor. AST: aspartate aminotransferase, ALT: serum alanine aminotransferase, γ-GTP: γ-glutamyl transpeptidase, Alb: albumin, T-bil: total bilirubin, PT-INR: international normalized ratio of prothrombin time.
(DOC)
Pediatric End-stage Liver Disease (PELD) score of patients. Alb: albumin, T-bil: total bilirubin, PT-INR: international normalized ratio of prothrombin time.
(XLS)
Acute cellular rejection episodes and Immunosuppression status. ACR: Acute Cellular Rejection.
(XLS)
Acknowledgments
We thank Sachiko Nishimura and Jun Nishimura for technical assistance, Dr. Shunzaburo Iwatsuki for helpful discussions, and Dr. David Douglas for language editing.
Funding Statement
This work was supported by Japan Society for the Promotion of Science (JSPS) KAKENHI, 19790926 and 24592013 (to Y.K.), 24590468 (to N.I.). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Starzl TE, Groth CG, Brettschneider L, Penn I, Fulginiti VA, et al. (1968) Orthotopic homotransplantation of the human liver. Ann Surg 168: 392–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Attia M, Silva MA, Mirza DF (2008) The marginal liver donor—an update. Transpl Int 21: 713–724. [DOI] [PubMed] [Google Scholar]
- 3. Jain A, Reyes J, Kashyap R, Dodson SF, Demetris AJ, et al. (2000) Long-term survival after liver transplantation in 4,000 consecutive patients at a single center. Ann Surg 232: 490–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Starzl TE, Klintman GBG, Porter KA, Iwatsuki S, Schroter GP (1981) Liver transplantation with use of cyclosporine A and prednisone. N Engl J Med 305: 266–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Starzl TE, Fung J, Venkataramman R, Todo S, Demetris AJ, et al. (1989) FK506 for human liver, kidney and pancreas transplantation. Lancet 334: 1000–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Calne RY, Rolles K, White DJ, Thiru S, Evans DB, et al. (1979) Cyclosporin A initially as the only immunosuppressant in 34 recipients of cadaveric organs: 32 kidneys, 2 pancreas, and 2 livers. Lancet 314: 1033–1036. [DOI] [PubMed] [Google Scholar]
- 7. Abbasoglu O (2008) Liver transplantation: yesterday, today and tomorrow. World J Gastroenterol 14: 3117–3122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mehrabi A, Fonouni H, Muller SA, Schmidt J (2008) Current concepts in transplant surgery: liver transplantation today. Langenbecks Arch Surg 393: 245–260. [DOI] [PubMed] [Google Scholar]
- 9. Evans HM, Kelly DA, McKiernan PJ, Hubscher S (2006) Progressive histological damage in liver allografts following pediatric liver transplantation. Hepatology 43: 1109–1117. [DOI] [PubMed] [Google Scholar]
- 10. Barber K, Blackwell J, Collett D, Neuberger J (2007) Life expectancy of adult liver allograft recipients in the UK. Gut 56: 279–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Blackburn EH (2000) Telomere states and cell fate. Nature 408: 53–56. [DOI] [PubMed] [Google Scholar]
- 12. Takubo K, Nakamura K, Izumiyama N, Furugori E, Sawabe M, et al. (2000) Telomere shortening with aging in human liver. J Gerontol A Biol Sci Med Sci 55: B533–536. [DOI] [PubMed] [Google Scholar]
- 13. Takubo K, Izumiyama-Shimomura N, Honma N, Sawabe M, Arai T, et al. (2002) Telomere lengths are characteristic in each human individual. Exp Gerontol 37: 523–531. [DOI] [PubMed] [Google Scholar]
- 14. Saretzki G, von Zglinicki T (2002) Replicative aging, telomeres, and oxidative stress. Ann N Y Acad Sci 959: 24–29. [DOI] [PubMed] [Google Scholar]
- 15. Aikata H, Takaishi H, Kawakami Y, Takahashi S, Kitamoto M, et al. (2000) Telomere reduction in human liver tissues with age and chronic inflammation. Exp Cell Res 256: 578–582. [DOI] [PubMed] [Google Scholar]
- 16. O'Sullivan JN, Bronner MP, Brentnall TA, Finley JC, Shen WT, et al. (2002) Chromosomal instability in ulcerative colitis is related to telomere shortening. Nat Genet 32: 280–284. [DOI] [PubMed] [Google Scholar]
- 17. Epel ES, Blackburn EH, Lin J, Dhabhar FS, Adler NE, et al. (2004) Accelerated telomere shortening in response to life stress. Proc Natl Acad Sci U S A 101: 17312–17315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Aida J, Yokoyama A, Izumiyama-Shimomura N, Nakamura K, Ishikawa N, et al. (2011) Alcoholics show reduced telomere length in the oesophagus. J Pathol 223: 410–416. [DOI] [PubMed] [Google Scholar]
- 19. Chkhotua A, Shohat M, Tobar A, Magal N, Kaganovski E, et al. (2002) Replicative senescence in organ transplantation-mechanisms and significance. Transpl Immunol 9: 165–171. [DOI] [PubMed] [Google Scholar]
- 20. Baerlocher GM, Rovo A, Muller A, Matthey S, Stern M, et al. (2009) Cellular senescence of white blood cells in very long-term survivors after allogeneic hematopoietic stem cell transplantation: the role of chronic graft-versus-host disease and female donor sex. Blood 114: 219–222. [DOI] [PubMed] [Google Scholar]
- 21. Harley CB, Futcher AB, Greider CW (1990) Telomeres shorten during ageing of human fibroblasts. Nature 345: 458–460. [DOI] [PubMed] [Google Scholar]
- 22. Allsopp RC, Vaziri H, Patterson C, Goldstein S, Younglai EV, et al. (1992) Telomere length predicts replicative capacity of human fibroblasts. Proc Natl Acad Sci U S A 89: 10114–10118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lansdorp PM, Verwoerd NP, van de Rijke FM, Dragowska V, Little MT, et al. (1996) Heterogeneity in telomere length of human chromosomes. Hum Mol Genet 5: 685–691. [DOI] [PubMed] [Google Scholar]
- 24. Meeker AK, Gage WR, Hicks JL, Simon I, Coffman JR, et al. (2002) Telomere length assessment in human archival tissues: combined telomere fluorescence in situ hybridization and immunostaining. Am J Pathol 160: 1259–1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ferlicot S, Youssef N, Feneux D, Delhommeau F, Paradis V, et al. (2003) Measurement of telomere length on tissue sections using quantitative fluorescence in situ hybridization (Q-FISH). J Pathol 200: 661–666. [DOI] [PubMed] [Google Scholar]
- 26. Aida J, Izumiyama-Shimomura N, Nakamura K, Ishikawa N, Poon SS, et al. (2008) Basal cells have longest telomeres measured by tissue Q-FISH method in lingual epithelium. Exp Gerontol 43: 833–839. [DOI] [PubMed] [Google Scholar]
- 27. McDiarmid SV, Merion RM, Dykstra DM, Harper AM (2004) Selection of pediatric candidates under the PELD system. Liver Transpl 10: S23–30. [DOI] [PubMed] [Google Scholar]
- 28. Cowles RA, Lobritto SJ, Ventura KA, Harren PA, Gelbard R, et al. (2008) Timing of liver transplantation in biliary atresia – results in 71 children managed by a multidisciplinary team. J Pediatr Surg 43: 1605–1609. [DOI] [PubMed] [Google Scholar]
- 29. Sugawara Y, Makuuchi M (1999) Technical advances in living-related liver transplantation. J Hepatobiliary Pancreat Surg 6: 245–253. [DOI] [PubMed] [Google Scholar]
- 30. Demetris AJ, Adeyi O, Bellamy CO, Clouston A, Charlotte F, et al. (2006) Liver biopsy interpretation for causes of late liver allograft dysfunction. Hepatology 44: 489–501. [DOI] [PubMed] [Google Scholar]
- 31. Takubo K, Aida J, Izumiyama N, Ishikawa N, Fujiwara M, et al. (2010) Chromosomal instability and telomere lengths of each chromosomal arm measured by Q-FISH in human fibroblast strains prior to replicative senescence. Mech Age Dev 131: 614–624. [DOI] [PubMed] [Google Scholar]
- 32. Yoshiya S, Shirabe K, Kimura K, Yoshizumi T, Ikegami T, et al. (2012) The causes, risk factors, and outcomes of early relaparotomy after living-donor liver transplantation. Transplantation 94: 947–952. [DOI] [PubMed] [Google Scholar]
- 33. Floreani A (2007) Liver Diseases in the Elderly: An Update. Dig Dis. 25: 138–143. [DOI] [PubMed] [Google Scholar]
- 34. Borchert D, Glanemann M, Mogl M, Langrehr JM, Neuhaus P (2005) Older liver graft transplantation, cholestasis and synthetic graft function. Transpl Int 18: 709–715. [DOI] [PubMed] [Google Scholar]
- 35. Singhal A, Sezginsoy B, Ghuloom AE, Hutchinson IV, Cho YW, et al. (2010) Orthotopic liver transplant using allografts from geriatric population in the United States: is there any age limit? Exp Clin Transplant 8: 196–201. [PubMed] [Google Scholar]
- 36. Campisi J (2001) From cells to organisms: can we learn about aging from cells in culture? Exp Gerontl 36: 607–618. [DOI] [PubMed] [Google Scholar]
- 37. Hornsby PJ (2002) Cellular senescence and tissue aging in vivo. J Gerontol A Biol Sci Med Sci 57: B251–256. [DOI] [PubMed] [Google Scholar]
- 38. Ishikawa N, Nakamura KI, Izumiyama N, Aida J, Sawabe M, et al. (2012) Telomere length dynamics in the human pituitary gland: robust preservation throughout adult life to centenarian age. Age 34: 795–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Paradis V, Youssef N, Dargere D, Ba N, Bonvoust F, et al. (2001) Replicative senescence in normal liver, chronic hepatitis C, and hepatocellular carcinomas. Hum Pathol 32: 327–332. [DOI] [PubMed] [Google Scholar]
- 40. Wiemann SU, Satyanarayana A, Tsahuridu M, Tillmann HL, Zender L, et al. (2002) Hepatocyte telomere shortening and senescence are general markers of human liver cirrhosis. Faseb J 16: 935–942. [DOI] [PubMed] [Google Scholar]
- 41. Aini W, Miyagawa-Hayashino A, Tsuruyama T, Hashimoto S, Sumiyoshi S, et al. (2012) Telomere shortening and karyotypic alterations in hepatocytes in long-term transplanted human liver allografts. Transpl Int 25: 956–966. [DOI] [PubMed] [Google Scholar]
- 42. Takubo K, Fujita M, Izumiyama N, Nakamura K, Ishikawa N, et al. (2010) Q-FISH analysis of telomere and chromosome instability in the oesophagus with and without squamous cell carcinoma in situ. J Pathol 221: 201–209. [DOI] [PubMed] [Google Scholar]
- 43. Sanada Y, Aida J, Kawano Y, Nakamura K, Shimomura N, et al. (2012) Hepatocellular Telomere Length in Biliary Atresia Measured by Q-FISH. World Journal of Surgery 36: 908–916. [DOI] [PubMed] [Google Scholar]
- 44. Nobori S, Shimizu A, Okumi M, Samelson-Jones E, Griesemer A, et al. (2006) Thymic rejuvenation and the induction of tolerance by adult thymic grafts. Proc Natl Acad Sci U S A 103: 19081–19086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Starzl TE, Murase N, Ildstad S, Ricordi C, Demetris AJ, et al. (1992) Cell migration, chimerism, and graft acceptance. Lancet 339: 1579–1582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Wege H, Brummendorf TH (2007) Telomerase activation in liver regeneration and hepatocarcinogenesis: Dr. Jekyll or Mr. Hyde? Curr Stem Cell Res Ther 2: 31–38. [DOI] [PubMed] [Google Scholar]
- 47. Nakajima T, Moriguchi M, Katagishi T, Sekoguchi S, Nishikawa T, et al. (2006) Premature telomere shortening and impaired regenerative response in hepatocytes of individuals with NAFLD. Liver Int 26: 23–31. [DOI] [PubMed] [Google Scholar]
- 48. Ohe H, Li Y, Nafady-Hego H, Kayo W, Sakaguchi S, et al. (2011) Minimal but essential doses of immunosuppression: a more realistic approach to improve long-term outcomes for pediatric living-donor liver transplantation. Transplantation 91: 808–810. [DOI] [PubMed] [Google Scholar]
- 49.Sanada Y, Kawano Y, Miki A, Aida J, Nakamura KI, et al.. (2014) Maternal grafts protect daughter recipients from acute cellular rejection after pediatric living donor liver transplantation for biliary atresia. Transpl Int. doi: 10.1111/tri.12273. [Epub ahead of print] [DOI] [PubMed]
- 50. Markus BH, Duquesnoy RJ, Gordon RD, Fung JJ, Vanek M, et al. (1988) Histocompatibility and liver transplant outcome. Does HLA exert a dualistic effect? Transplantation 46: 372–377. [PMC free article] [PubMed] [Google Scholar]
- 52. Veldhuis JD (2008) Aging and hormones of the hypothalamo-pituitary axis: gonadotropic axis in men and somatotropic axes in men and women. Ageing Res Rev. 7: 189–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Nair KS (2005) Aging muscle. Am J Clin Nutr. 81: 953–63. [DOI] [PubMed] [Google Scholar]
- 54. Nakamura K, Izumiyama-Shimomura N, Sawabe M, Arai T, Aoyagi Y, et al. (2002) Comparative analysis of telomere lengths and erosion with age in human epidermis and lingual epithelium. J Invest Dermatol. 119: 1014–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Laboratory data of recipients and donors at biopsy. Lower NTCR group: Median NTCRs of the recipient hepatocyte was significantly lower than those of the donor. Comparable NTCR group: Median NTCRs of the recipient hepatocyte was neither significantly lower nor higher than those of the donor. AST: aspartate aminotransferase, ALT: serum alanine aminotransferase, γ-GTP: γ-glutamyl transpeptidase, Alb: albumin, T-bil: total bilirubin, PT-INR: international normalized ratio of prothrombin time.
(DOC)
Pediatric End-stage Liver Disease (PELD) score of patients. Alb: albumin, T-bil: total bilirubin, PT-INR: international normalized ratio of prothrombin time.
(XLS)
Acute cellular rejection episodes and Immunosuppression status. ACR: Acute Cellular Rejection.
(XLS)