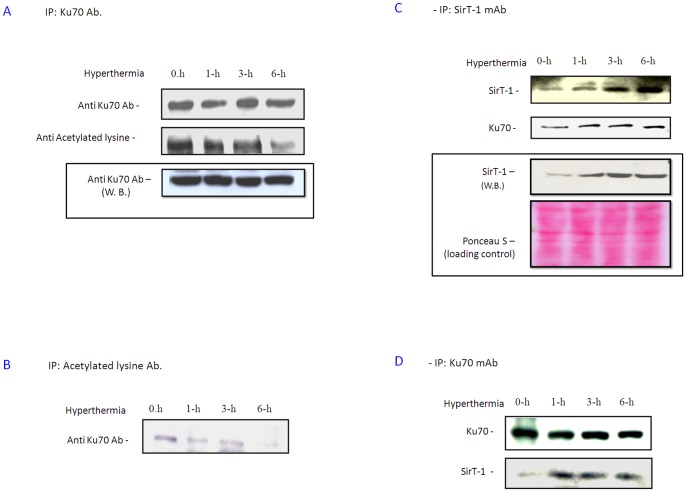
Figure 4. Hyperthermia treatment reduced Ku70 acetylation.
A. Ku70 was immunoprecipitated from equal protein amounts (3 mg) of PC-10 cell lysat after hyperthermia at indicated time. Ku70 and acetyl Lysine were detected in the immunoprecipitant. Total Ku70 showed no change after 6 h hyperthermia (in put) but acetylated Ku70 was decreased. B. All acetylated proteins were immunoprecipitated, fractionated, blotted and Ku70 was detected in the blot. Bands indicated acetylated Ku70 became fainter with increasing hyperthermia time. C. Hyperthermia enhanced both Ku70 expression and Ku70/SirT-1 binding. SirT-1 was immunoprecipitated from PC-10 after hyperthermia (0–6 h). Blot indicates SirT-1 up-regulation (in put; lower panel) and enhanced binding to Ku70, in the same blot, was up-regulated (upper panels). D. Similar hyperthermia treatment in H1299. Total Ku70 was precipitated. Ku70 and SirT-1 were detected in the immunoprecipitant by Western analysis. SirT-1/Ku70 binding was increased, indicating an enhanced Ku70 deacetylation in lung cancer cells by hyperthermia.