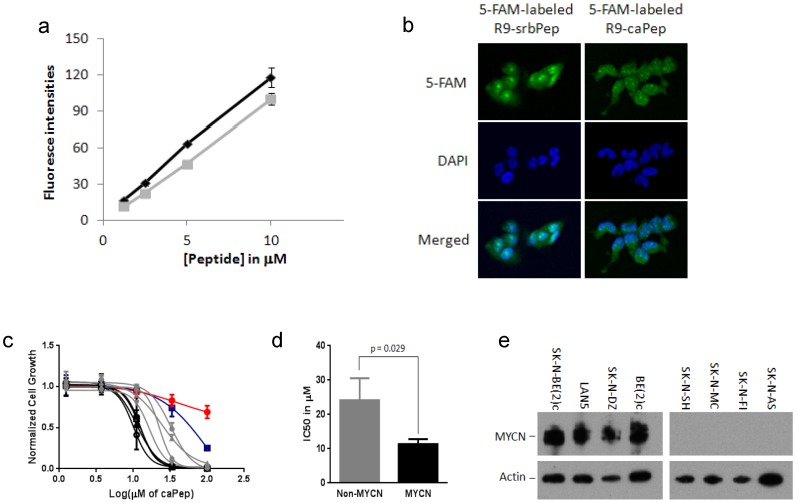
Figure 1. Permeability and selective cytotoxicity of R9-caPep in NB cells.
a) SK-N-DZ NB cells were treated in triplicates by various concentrations of 5-FAM labeled R9-caPep (gray) or R9-srbPep (dark). After cells were treated by trypsin and washed, their fluorescence intensities were determined by flow cytometry. The median fluorescence intensities for triplicate cell populations under each treatment condition were averaged and graphed plus/minus standard deviations. b) Cells treated by 10 µM 5-FAM labeled R9-caPep or R9-srbPep were examined by confocal microscopy. The nuclear areas were indicated by DAPI staining. c) Four NB cell lines with MYCN amplification (in black), four NB cell lines without MYCN amplification (in grey), human PBMCs (red cycles), and human neural crest stem cell line 7SM0032 (blue squares) were cultured in the presence of various concentrations of the R9-caPep for 72 h. Cell growth was determined by a CellTiter-Glo luminescence assay (Promega). Cells cultured in the absence of the R9-caPep were used as control. Luminescent signals in triplicates normalized to the control for each cell line were averaged and graphed plus/minus standard deviations. Black filled squares: LAN5, black triangles: SK-N-DZ, black circles: SK-N-BE(2)c, black empty squares: BE(2)c, grey diamond: SK-N-AS, grey circle: SK-N-SH, grey triangle: SK-N-MC, and grey square: SK-N-FI. d) The IC50s of the peptide on cell lines with or without MYCN amplification, determined by non-linear fit of Prism 6 (GraphPad Software, La Jolla, CA), were averaged and graphed plus/minus standard deviations. e) Total cell lysates were extracted from the indicated cell lines. The expression of MYCN and actin in these cell lines were determined by western blot.