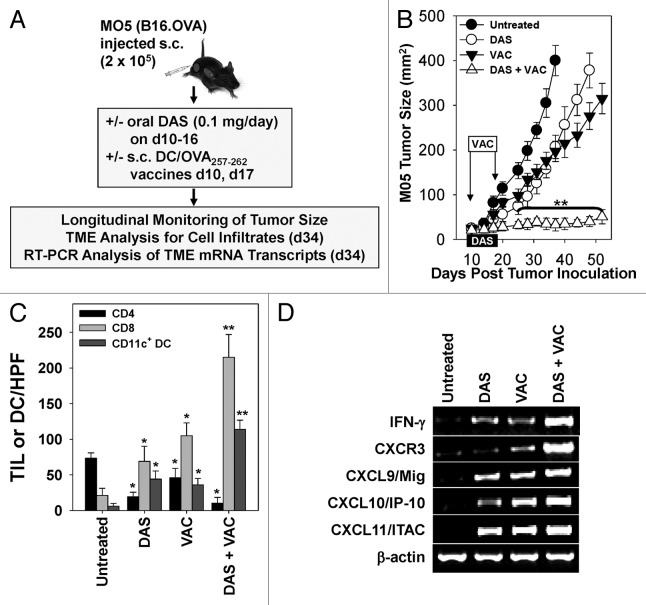
Figure 2. Combination dasatinib + OVA peptide-based dendritic cell vaccination therapy yields superior antitumor efficacy and immune cell recruitment into the tumor microenvironment vs. either monotherapy. (A–D) C57BL/6 mice bearing subcutaneous M05 melanomas (n = 7 mice/group) established 10 d prior were left untreated or were treated with either 0.1 mg/day dasatinib (DAS) administered by oral gavage for 7 consecutive days, contralateral s.c. vaccination (VAC) consisting of 106 OVA257–264 peptide-pulsed dendritic cells (DCs) genetically modified to overexpress IL-12, or a combination of DAS + VAC. (B) Animals were monitored for tumor growth every 3–4 d using external calipers and estimated tumor volume calculated as the product of the orthogonal measurements. Data are the mean ± SD tumor volume per group. (C) On day 34, 2 mice/group were sacrificed and cryo-preserved tumor sections analyzed by immunofluorescence staining and fluorescence microscopy for CD4+ T cell, CD8+ T cell, and CD11c+ DC content. The numbers of CD4+ and CD8+ tumor-infiltrating lymphocytes (TILs) or DC are reported as the mean ± SD of the indicated cells over 10 high-power fields (HPF). (D) Total tumor mRNA purified from representative tumors from the indicated treatment group was analyzed by RT-PCR for expression of the indicated Type-1-associated cytokine, chemokine, or chemokine receptor transcript. Representative data from 1 of 3 independent experiments are depicted. Statistical analyses were performed by Student’s t test or 1-way ANOVA; *P < 0.05 vs. untreated (t test) and **P < 0.05 vs. all other groups (ANOVA).

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.