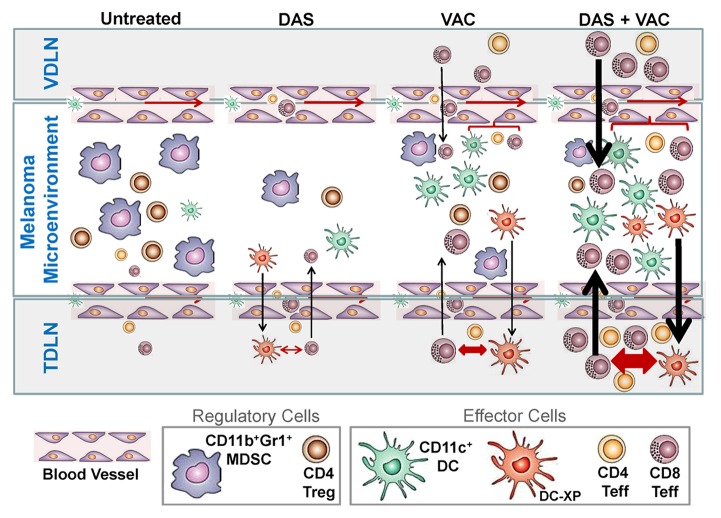
Figure 5. Proposed mechanism for the superior antitumor action of combined DAS + VAC immunotherapy. We propose that DAS monotherapy exerts an antagonistic effect on tumor-associated myeloid-derived suppressor cells (MDSCs) and regulatory T cells (Tregs) manifesting in reduced numbers of such regulatory cells in the melanoma microenvironment. This may be the result of reduced recruitment of MDSCs or Tregs due to reduced levels of relevant recruiting chemokines such as CXCL12, or due to dasitinib (DAS)-mediated inhibition of MDSC or Treg expansion and differentiation within the tumor microenvironment. Removal of such suppression may allow for improved recruitment and function of CD11c+ dendritic cells (DCs) and the effector T (Teff) cells that are cross-primed (by DC-XP) in the tumor-draining lymph node (TDLN). Vaccination (VAC) monotherapy drives anti-OVA CD8+ T cell responses in the vaccine-site draining lymph node (VDLN) and the subsequent trafficking of such effector cells into the tumor area results in locoregional production of IFNγ that stimulates production of CXCR3+ chemokines (such as CXCL9–11) allowing for the corollary recruitment of additional Type-1 CXCR3+ tumor-infiltrating lymphocytes (TILs), a portion of which are the result of cross-priming events in the TDLN. Treatment with DAS + VAC provides an optimal combination therapy that stimulates Type-1 immunity (both T cell and DC) and mitigates immunosuppression associated with MDSCs and Tregs. Re-iterated cycles of IL-12hiIL-10lowCD11c+ DC cross-priming of CD8+ T cells in TDLN results in the rapid broadening of the antitumor T cell repertoire, allowing for effective immune-mediated control of both melanoma cells and tumor-associated stromal cell populations, such as EphA2+ vascular endothelial cells, and superior therapeutic efficacy. Note: Larger symbol sizes and bolder lines infer greater numbers or strength of responses.

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.