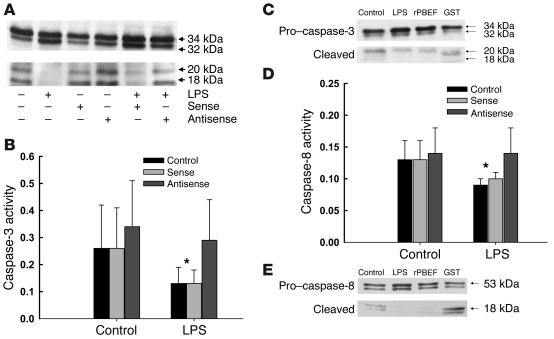
Figure 5.
PBEF inhibits the cleavage and catalytic activity of caspase-3 and blocks the activity of caspase-8. (A) Caspase-3 is cleaved to yield active 18- to 20-kDa fragments in constitutively apoptotic neutrophils; LPS inhibits the activational cleavage of this effector caspase, as evaluated by Western blot analysis following 6 hours of in vitro culture. PBEF antisense prevented the inhibitory effects of LPS, while the sense control did not. Blot shown is representative of three separate studies. (B) Caspase-3 activity, measured colorimetrically in arbitrary units as the cleavage of the tetrapeptide Ac-DEVD-pNA was also reduced in neutrophils following exposure to LPS; PBEF antisense, but not the sense control, prevented this reduction in caspase-3 activity. Results are means ± SD of six separate studies; *P < 0.05 versus control levels or activity levels in neutrophils treated with PBEF antisense. (C) Cleavage of pro_caspase-3 to active caspase-3 (20 kDa) was reduced in neutrophils incubated with LPS (1 μg/ml) or rPBEF (50 ng/ml); Western blot is representative of 3 separate experiments. (D) Caspase-8 activity, measured colorimetrically in arbitrary units as the cleavage of the caspase-8 tetrapeptide target Ac-IETD-pNA was reduced by exposure to LPS and restored by pretreatment of neutrophils with PBEF antisense. Results are means ± SD of six separate studies; *P < 0.05, control or sense-treated cells versus antisense-treated neutrophils or neutrophils cultured in the absence of LPS. (E) Cleavage of pro_caspase-8 (53 kDa) to its active form (18 kDa) was also inhibited by exposure of neutrophils to LPS or rPBEF; Western blot is representative of 3 separate experiments.