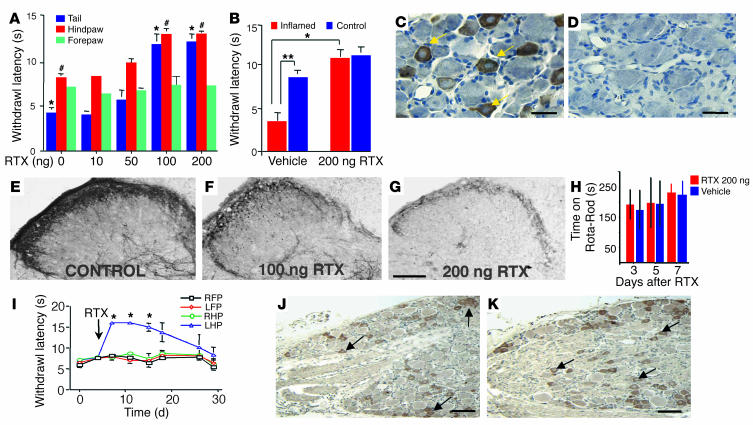
Figure 3.
Intrathecal (multiganglionic) or peripheral RTX attenuates thermal nociception and inflammatory hyperalgesia. (A) Dose_response to lumbar intrathecal administration of RTX (n = 50, 10 per group; *,#P < 0.05). Doses lower than 50 ng were without effect. Robust analgesia was obtained for the tail and hindpaws at doses of 100 and 200 ng. (B) Carrageenan inflammatory hyperalgesia (n = 4 per group; ** P < 0.05) is reversed after 200 ng intrathecal RTX (*P < 0.05). No analgesic effect was seen in forepaws (A), correlating with retention of TRPV1-IR neurons in the cervical ganglia (C) and their loss in lumbosacral ganglia (D). Dose-related reduction of CGRP-IR, a neuropeptide expressed by nociceptive afferent terminals, in the lumbar spinal cord at 3 days after administration of RTX (E_G). No significant effect on locomotor performance occurs as examined using an accelerating Rota-Rod (H). Peripheral left hindpaw (LHP) administration of 100 ng RTX yields unilateral and reversible (about 20 days) thermal analgesia (*ANOVA with Scheffe’s post hoc test; P < 0.005) (n = 5 per group). RHP, right hindpaw; FP, forepaw. (I) Retention of TRPV1-IR neurons (arrows) in L5 DRGs from RTX-injected (J) and noninjected (K) hindpaws. Cell counts of TRPV1-IR perikarya showed no significant alteration between left and right lumbar ganglia (see Results). Bars: 50 ∝m (C and D); 100 ∝m (J and K); 300 ∝m (E_G).