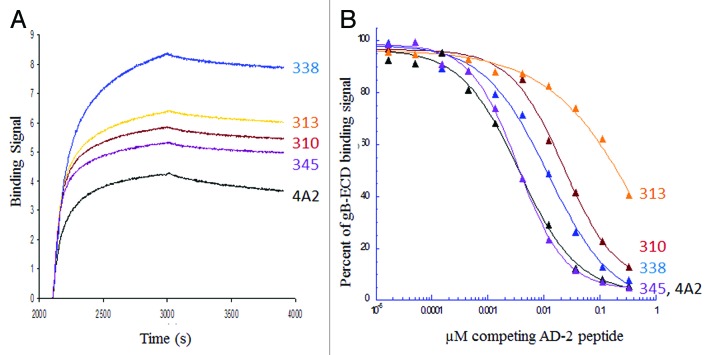
Figure 5. Binding of selected high-affinity human CMV antibodies to the neutralizing AD-2 epitope alone and in the context of glycoprotein B. (A) Sensorgrams of 100 nM IgG (mAb 310, 313, 338, 345 and 4A2) binding to biotinylated AD-2 peptide captured on streptavidin probes. (B) Competitive binding ELISA using a constant amount of the same panel of mAb IgG pre-incubated with increasing amounts of AD-2 peptide for 30 min prior to binding plate-coated gB. Concentrations of IgG were normalized among the antibodies to give an OD = 1 when binding 0.5 µg/ml gB for 1 h in the absence of any competing peptide. High-affinity antibodies cloned from memory B cells of naturally infected humans with HCMV, such as mAbs 310, 313 and 338, demonstrate additional contacts with gB outside of the AD-2 region causing them to bind gB more strongly in the presence of competing peptide.

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.