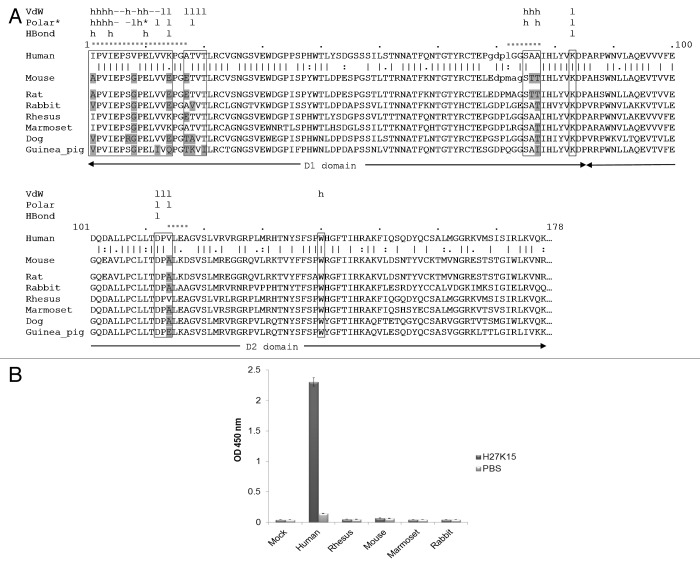
Figure 5. Cross species experiments. (A) Sequence alignment of the D1-D2 domains of CD115 from different species. Predicted interaction sites of the epitope are highlighted in boxes. Residues of the epitope that differ from the human sequence are indicated in gray. The unresolved residues of the human and mouse crystal structures loops are represented in lower case. Interaction types are shown above the sequence, VdW (van der Waals), * indicates an electrostatic polar interaction. The mAb chains involved in interactions are also mentioned: h, heavy chain; l, light chain; -, both chains. Dashed segments indicate the human fragments used for the chimeric CD115 constructs. (B) Double sandwich ELISA experiment with H27K15 mAb. The transiently expressed CD115D1-D5 constructs from various species fused with an 8 histidine tag were captured with a coated anti-penta histidine antibody, then incubated in the presence of either biotinylated H27K15 (dark gray bars) or PBS (light gray bars). Bound H27K15 was revealed by streptavidin-HRP followed by TMB incubation.

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.