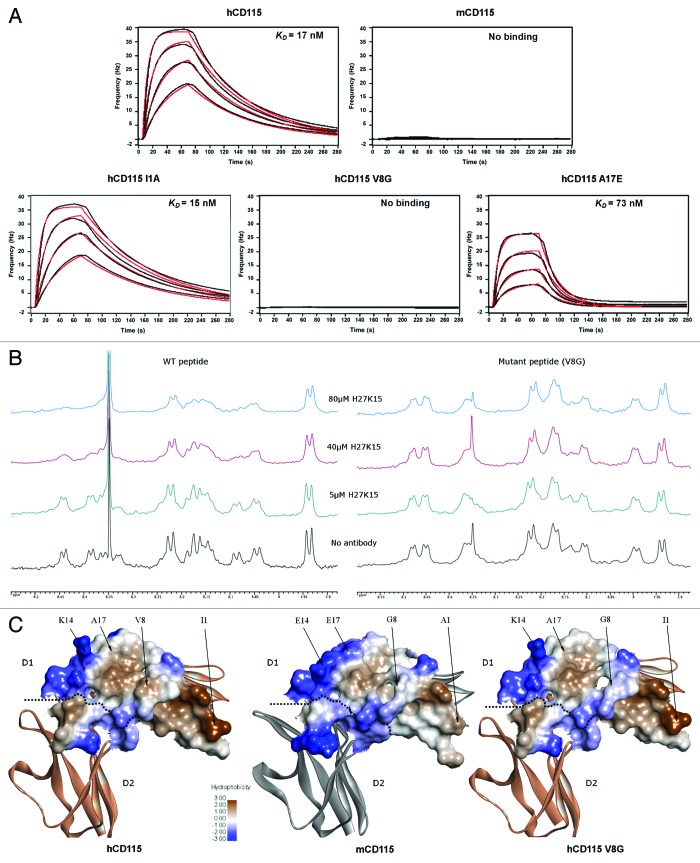
Figure 6. In vitro binding experiments of the H27K15 mAb on various CD115 constructs. (A) Quartz Crystal Microbalance affinity profiles of the immobilized H27K15 mAb in association with hCD115D1-D5, mCD115D1-D5 and the hCD115D1-D5 I1A, V8G and A17E single-point mutants. Raw data (black); model fitting (red). Kinetics parameters are reported in Table 2. (B) HN region of the 1D 1H NMR spectra of 250 µM peptide with the addition of increasing amounts of H27K15 antibody. Left panel, spectra of the WT (hCD115) 23-mer peptide. Right panel, spectra of the V8G mutant 23-mer peptide. (C) hydrophobicity of the interaction surface of the hCD115 and mCD115 crystal structures and the hCD115 V8G variant model based on the hCD115 crystal structure. Positions of the 4 residues of interest are indicated. Hydrophobicity plot and visualization were generated with Discovery Studio v3.5.

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.