Abstract
Background and Objectives
Alcohol abuse complicates treatment of HIV disease and is linked to poor outcomes. Alcohol pharmacotherapies, including disulfiram (DIS), are infrequently utilized in co-occurring HIV and alcohol use disorders possibly related to concerns about drug interactions between antiretroviral (ARV) medications and DIS.
Method
This pharmacokinetics study (n=40) examined the effect of DIS on efavirenz (EFV), ritonavir (RTV), or atazanavir (ATV) and the effect of these ARV medications on DIS metabolism and aldehyde dehydrogenase (ALDH) activity which mediates the DIS-alcohol reaction.
Results
EFV administration was associated with decreased S-Methyl-N-N-diethylthiocarbamate (DIS carbamate), a metabolite of DIS (p=0.001) and a precursor to the metabolite responsible for ALDH inhibition, S-methyl-N,N-diethylthiolcarbamate sulfoxide (DETC-MeSO). EFV was associated with increased DIS inhibition of ALDH activity relative to DIS alone administration possibly as a result of EFV-associated induction of CYP 3A4 which metabolizes the carbamate to DETC-MeSO (which inhibits ALDH). Conversely, ATV co-administration reduced the effect of DIS on ALDH activity possibly as a result of ATV inhibition of CYP 3A4. DIS administration had no significant effect on any ARV studied.
Discussion/Conclusions
ATV may render DIS ineffective in treatment of alcoholism.
Future Directions
DIS is infrequently utilized in HIV-infected individuals due to concerns about adverse interactions and side effects. Findings from this study indicate that, with ongoing clinical monitoring, DIS should be reconsidered given its potential efficacy for alcohol and potentially, cocaine use disorders, that may occur in this population.
Keywords: disulfiram, alcohol abuse, efavirenz, ritonavir, atazanavir, drug interactions, aldehyde dehydrogenase
Introduction
Disulfiram (DIS) (Antabuse®) is a U.S. Food and Drug Administration (FDA) approved pharmacotherapy for the treatment of alcohol dependence. DIS is a relatively irreversible inhibitor of sulfhydryl-containing enzymes (1). The target enzyme for the pharmacologic effect of DIS in the treatment of alcohol addiction is aldehyde dehydrogenase (ALDH). This enzyme converts acetaldehyde to acetate in alcohol metabolism. The increased concentration of acetaldehyde after alcohol ingestion in the presence of DIS is responsible for the DIS-alcohol reaction. This reaction is characterized by flushing, weakness, nausea, tachycardia, and in some instances, hypotension (2). In addition to its use as a deterrent to alcohol consumption, DIS has shown promise as a potential pharmacotherapy for cocaine abuse (3). Clinical trials have shown decreases in cocaine and alcohol use with DIS 250 mg daily administration (4) while human laboratory studies have shown significant decreases in cocaine “high” and “rush” when cocaine was administered by the intravenous route in a double-blind, randomized study in which either DIS 0, 62.5 mg or 250 mg daily was administered chronically (5).
Substance abuse is common in those with HIV infection (6, 7) and the availability of addiction pharmacotherapy for this population is important. For example, the clinical benefit of opioid therapies including methadone and buprenorphine to treat opioid dependence, often in injection drug users who have contracted HIV infection through high risk injection practices, has been clearly demonstrated (8). However, medications to treat alcoholism are used infrequently although alcohol abuse is a significant problem complicating the treatment of HIV disease and has been linked to poor HIV outcomes (9–11). The potential of DIS for adverse drug-drug interactions with antiretroviral medications (ARV) is a significant concern that could be a factor in diminishing consideration of use of this medication in those with alcoholism and HIV disease.
DIS must be bioactivated through a series of intermediates, ultimately forming S-methyl-N,N-diethylthiolcarbamate sulfoxide (DETC-MeSO), the metabolite proposed to be responsible for the in vivo inhibition of ALDH (12, 13). Bioactivation of DIS to the active metabolite associated with the DIS-alcohol reaction, DETC-MeSO, occurs principally via cytochrome P450 (CYP) 3A4/5, with contributions by CYP1A2, -2A6 and -2D6 (14, 15). Inhibition by DIS of CYP450 enzymes, mainly 2E1 (16, 17) and 1A2 (18, 19), and demethylation function has been reported (20). Therefore, this study investigated any potential effect on CYP450 enzymes by DIS that might alter the metabolism of ARV, specifically efavirenz (EFV), ritonavir (RTV), and atazanavir (ATV) since these ARV are substrates of CYP3A4/5 and -2B6. Also important was the determination of whether DIS would be efficacious as a treatment for alcohol dependence when co-administered with ARV that could potentially alter DIS metabolism. For example, EFV has been reported to induce CYP3A4 activity (21, 22) while RTV is a potent inhibitor of CYP3A4 (23). Therefore, the effects of ARV administration on DIS metabolism were also examined.
Methods
Forty individuals participated in this project composed of four separate pharmacokinetics study components with 10 participants enrolled in each component which included determination of pharmacokinetics for 1. DIS alone, 2. DIS/EFV, 3. DIS/RTV, and 4. DIS/ATV. The study was reviewed and approved by the Institutional Review Board at the University of California San Francisco (UCSF) and is registered at ClinicalTrials.gov (NCT00878306). Participants were healthy subjects with health status determined by medical history and physical examination, psychological testing (Mini-International Neuropsychiatric Interview (24), laboratory testing, and cardiogram which were within normal ranges at baseline (Table 1). Participants were taking no other medications that might impact CYP 450 function. All participants were confirmed to have no evidence of HIV infection (by HIV antibody and HIV viral load tests). Participants were tested for ALDH activity which is a marker of DIS ability to induce a DIS-alcohol reaction (i.e.: whether DIS would be expected to be effective as a deterrent to alcohol use) and underwent pharmacokinetics studies in which a within-subjects analysis was used to determine the effect of ARV combined with DIS on ALDH activity. A between-subjects analysis was used to examine the effect of ARV on the DIS metabolite, S-Methyl-N-N-diethylthiocarbamate (DIS carbamate), and the effect of DIS on ARV.
Table 1.
Sample Characteristics
| No ARV N = 10 |
Ritonavir N = 10 |
Efavirenz N = 10 |
Atazanavir N = 10 |
|
|---|---|---|---|---|
| Age (yrs) | 38.1 (4.2)# | 43.4 (2.5) | 44.9 (4.0) | 33.8 (4.4) |
| Weight (kg) | 75.1 (4.9) | 79.7 (6.1) | 76.6 (5.5) | 87.4 (5.9) |
| Female | 6 [60%] | 3 [30%] | 4 [40%] | 3 [30%] |
| Race: | ||||
| African-American | 2 [20%] | 3 [30%] | 2 [20%] | 3 [30%] |
| Caucasian | 8 [80%] | 7 [70%] | 8 [80%] | 3 [30%] |
| Other | 0 [0%] | 0 [0%] | 0 [0%] | 4 [40%] |
| Nicotine Use (packs/day) | 0.0 (0.0) | 0.1 (0.1) | 0.1 (0.1) | 0.0 (0.0) |
| AST (U/L) | ||||
| Normal Range: 10–41 U/L | ||||
| Baseline | 25.1 (2.1) | 28.9 (2.4) | 27.3 (1.8) | 24.1 (1.8) |
| ARV Alone | 25.1 (0.8) | 32.9 (7.3) | 21.7 (1.7) | |
| DIS 250 mg | 27.0 (2.4) | 22.9 (0.9) | 35.0 (8.7) | 29.1 (6.8) |
| ALT (U/L) | ||||
| Normal Range: 7–35 U/L | ||||
| Baseline | 23.3 (2.9) | 25.4 (2.7) | 23.9 (2.5) | 21.9 (4.1) |
| ARV Alone | 20.4 (2.1) | 23.6 (3.0) | 19.9 (2.4) | |
| DIS 250 mg | 24.4 (3.6) | 20.2 (1.8) | 37.2 (10.1) | 26.8 (5.2) |
| ALK Phosphate (U/L) | ||||
| Normal Range: 42–98 U/L | ||||
| Baseline | 73.5 (6.2) | 71.8 (4.3) | 71.9 (4.3) | 85.1 (5.3) |
| ARV Alone | 65.8 (4.5) | 62.5 (6.3) | 82.2 (5.3) | |
| DIS 250 mg | 76.3 (7.1) | 67.5 (4.1) | 80.1 (5.0) | 86.6 (5.2) |
| Total Bilirubin (mg/dL) | ||||
| Normal Range: 0.1–1.2 mg/dL | ||||
| Baseline | 0.6 (0.1) | 0.7 (0.1) | 0.7 (0.1) | 0.6 (0.1) |
| ARV Alone | 0.5 (0.0) | 0.4 (0.0) | 2.3 (0.4)* | |
| DIS 250 mg | 0.6 (0.1) | 0.4 (0.0) | 0.4 (0.0) | 2.0 (0.5)* |
| Total Protein (g/dL) | ||||
| Normal Range: 6.4–8.3 g/dL | ||||
| Baseline | 7.4 (0.2) | 7.1 (0.1) | 7.2 (0.1) | 7.4 (0.1) |
| ARV Alone | 6.6 (0.1) | 6.8 (0.1) | 6.9 (0.1) | |
| DIS 250 mg | 7.1 (0.1) | 6.6 (0.1) | 7.0 (0.2) | 7.1 (0.1) |
| ECG QTc (ms) | ||||
| Baseline | 420.4 (5.4) | 409.0 (4.7) | 415.1 (4.8) | 404.5 (5.0) |
| ARV Alone | 410.6 (5.6) | 415.3 (5.6) | 384.9 (6.8) | |
| DIS 62.5 mg | 421.8 (3.9) | 409.6 (5.6) | 407.0 (8.8) | 383.8 (5.9) |
| DIS 250 mg | 420.8 (6.5) | 403.7 (6.3) | 403.1 (6.0) | 380.4 (7.1) |
Mean (SE), [ ] percent of sample affected
p ≤0.01
Other Race includes Hispanic, Native American, Pacific Islander and Multi-Racial individuals
Each study component was conducted independently and was open-label to increase the safety of the study participants. Participants in the DIS alone group received DIS 62.5 mg daily for 4 days followed by a pharmacokinetics study over 10 hours. Participants were then placed on DIS 250 mg daily and received this medication for 4 days followed by another pharmacokinetics study. Blood samples for DIS alone pharmacokinetics studies were collected at 0, 0.5, 1, 2, 4, 6, 8, and 10 hours. Volunteers who participated in the DIS/ARV studies were assigned to receive clinical doses of either EFV 600 mg daily for 10 days or RTV 200 mg daily for 8 days or ATV 400 mg daily for 8 days. Upon completion of the dosing period for the ARV, either a 12 hour (RTV) or 24 hour (ATV or EFV) pharmacokinetics study was undertaken in which ARV samples were collected at 0, 0.5, 1, 1.5, 2, 4, 6, 8, 12 and 24 hours (the latter sample for EFV and ATV studies). Once the ARV pharmacokinetics study was completed, participants began a 4 day course of DIS 62.5 mg daily with continued ARV medication followed by a second pharmacokinetics study in which both DIS and the ARV of interest were sampled as previously described. A four day washout of DIS with continued ARV administration was followed by a dosing period of 4 days with DIS 250 mg daily. All medication ingestion was observed by study staff with the exception of EFV which is generally given in the evening when used for HIV treatment. Four days prior to the pharmacokinetics studies, EFV dosing was changed to mornings and all dosing was observed. The final pharmacokinetics study of ARV and DIS 250 mg was undertaken as described above. Blood samples for ALDH were collected at baseline (prior to receiving any study medications) and following completion of study medication observed dosing (e.g.: Day 4 following DIS 62.5 mg daily and Day 4 following DIS 250 mg daily dosing for DIS alone and similarly following DIS administration in combination with ARV medications). Each ALDH sample was collected prior to DIS or DIS + ARV dosing at the time of the pharmacokinetics study. Adverse symptoms were recorded for all participants using an Adverse Symptoms Checklist (25) that queried for a wide range of adverse experiences including changes in energy, gastrointestinal symptoms, central nervous system effects, genitourinary symptoms, and other somatic complaints scored for severity on an ordinal scale (0–3, with 0=not present, 1=mild, 2=moderate, and 3=severe, maximum possible score=87). These ratings were administered at baseline, following DIS administration, following ARV administration, and following administration of ARV and DIS concomitantly. Upon completion of study procedures, participants received a final physical examination and laboratory testing to assure that no clinically significant changes had occurred.
Analytical Assays
Sample Processing
All blood samples were collected in heparinized 6 ml vacutainer tubes. For drug concentration determination, plasma was separated by centrifugation and stored at −70°C until shipment. Plasma samples were shipped on dry ice to the University at Buffalo Translational Pharmacology Research Core and kept at −70°C until assay. Samples were thawed prior to assays which were conducted as described below. Blood samples for ALDH determination were collected in heparinized vacutainer tubes and shipped overnight on a cold pack to the flow cytometery laboratory at Roswell Park Cancer Institute for analysis. The blood samples were processed for mononuclear cells using histopaque.
ALDH assay
ALDH activity in peripheral blood mononuclear cells was measured using ALDEFLUOR™ Kits. These kits are routinely used to identify stem and progenitor cells expressing high levels of ALDH (26). Total and control ALDH measurements were performed in triplicate. Cytofluorometric analysis was performed using a FACSCanto II (BD BioSciences, San Jose, CA) flow cytometer equipped with 408, 488, and 640 nm lasers. Monocytes were gated and the net ALDH activity determined by subtracting control from total activity.
S-Methyl-N-N-diethylthiocarbamate (DIS carbamate) and Antiretroviral Assays
DIS carbamate concentrations were determined using ultra performance liquid chromatography coupled to electrospray tandem mass spectrometry (27). S-Methyl-N-N-diethylthiocarbamate reference standard (lot number ELZ-125-3, 98% purity) was used to prepare calibration standards and quality controls and was supplied by Toronto Research Chemicals. Ritonavir, atazanavir and efavirenz were quantified using previously published simultaneous high-performance liquid chromatography (HPLC) assays (28, 29).
Pharmacokinetics and Statistical Analysis
Plasma DIS metabolite and ARV pharmacokinetics were evaluated for each subject. Standard non-compartmental methods were used to estimate pharmacokinetic parameters including area under the concentration-time curve (AUCtau,), maximum plasma concentration (Cmax), time of Cmax(Tmax), elimination half life (T1/2) and oral clearance (CLss/F), where F is the oral bioavailability. Tmax was estimated by inspection of the raw data (Phoenix 64, WinNonlin 6.3)
The Kruskal-Wallis test was used to determine the significance of the differences in ARV pharmacokinetic parameters (e.g.: AUC, Cmax, Cmin) in the absence or presence of DIS 62.5 and DIS 250 mg/d. Determination of differences in effects of ARV on DIS intermediary metabolite, DIS carbamate, pharmacokinetics parameters were also examined by Kruskal-Wallis test. ALDH activity was analyzed using a within-subjects approach by paired t-test with comparison of baseline ALDH and ALDH activity following DIS or DIS/ARV administration. All differences were considered statistically significant if the p value was ≤ 0.05 (two-tailed). Comparisons of participant characteristics were made by one-way ANOVA.
Results
Study Participants
There were no significant differences in age (range 34–45 years), weight (75–87 kg), gender (samples ranged from 30–60% women), or cigarette use between any of the groups (0–0.1 packs per day) (Table 1). All samples were comprised of healthy subjects and no diagnoses of medical, mental, or substance use disorders were identified on completion of screening procedures. Laboratory indices of hepatic function (alanine aminotransferase (ALT), aspartate aminotransferase (AST), alkaline phosphatase, and total protein) remained within normal ranges throughout the study period including baseline values, following ARV administration, DIS administration and combined ARV and DIS administration (Table 1). Total bilirubin was significantly increased following ATV administration alone and in combination with DIS. Cardiograms were obtained at baseline and following DIS administration, ARV administration or combined administration of ARV and DIS. There was no evidence for clinically significant changes in QT interval under any study condition (Table1). Administration of DIS, ARV, or DIS concurrently with any of the ARV produced no significant changes in adverse symptoms from those reported at baseline.
Pharmacokinetic results
Effect of DIS on ARV
Table 2 A–C shows pharmacokinetic parameters for each of the ARV alone or concurrent with either dose of DIS for 4 days. Neither dose of DIS had a significant effect on any of the pharmacokinetic parameters examined including total exposure to ARV over time (AUCtau), clearance, maximum plasma concentration (Cmax), time to maximum plasma concentration (Tmax), and elimination half-life.
Table2.
Effect of Disulfiram on Antiretroviral Medication Pharmacokinetics
| A. Ritonavir (RTV) pharmacokinetics during disulfiram co-administration | ||||
|---|---|---|---|---|
| Parameter | RTV | RTV/DIS62.5 | RTV/DIS250 | p |
| AUCtau(h*ng/ml) | 9369 (978) | 10899 (1069) | 10960 (1343) | 0.715 |
| Clss/F (ml/h) | 22977 (1995) | 19992 (2020) | 20501 (2161) | 0.714 |
| Cmax (ng/ml) | 1399 (151) | 1669 (243) | 1758 (248) | 0.568 |
| Tmax (h) | 5.0 (1.5–6.0) | 4.0 (1.0–6.0) | 4.0 (2.0–6.0) | 0.332 |
| T1/2 (h) | 3.2 (0.5) | 2.9 (0.2) | 3.0 (0.3) | 0.798 |
| B. Atazanavir (ATV) pharmacokinetics during disulfiram co-administration | ||||
|---|---|---|---|---|
| Parameter | ATV | ATV/DIS62.5 | ATV/DIS250 | p |
| AUCtau(h*ng/ml) | 21895 (3407) | 25411 (3011) | 23127 (3030) | 0.698 |
| Clss/F (ml/h) | 23515 (4112) | 18586 (2867) | 21250 (3538) | 0.698 |
| Cmax (ng/ml) | 2823 (414) | 3602 (409) | 3571 (467) | 0.426 |
| Tmax (h) | 4.0 (1.5–6.0) | 2.0 (1.5–4.0) | 2.0 (1.5–4.0) | 0.342 |
| T1/2 (h) | 8.3 (1.1) | 7.8 (0.8) | 7.4(0.7) | 1.939 |
| C. favirenz (EFV) pharmacokinetics during disulfiram co–administration | ||||
|---|---|---|---|---|
| Parameter | EFV | EFV/DIS62.5 | EFV/DIS250 | p |
| AUCtau(h*µg/ml) | 166.5 (45.4) | 166.3 (57.6) | 234.9 (118.7) | 0.882 |
| Clss/F (l/h) | 10.4 (1.0) | 11.2 (1.1) | 11.3 (1.0) | 0.517 |
| Cmax (µg/ml) | 4.6 (0.5) | 4.8 (0.9) | 4.4 (0.5) | 0.983 |
| Tmax (h) | 4.0 (1.0–12.0) | 4.0 (2.0–8.0) | 4.0 (2.0–8.0) | 0.862 |
| T1/2 (h) | 29.3 (4.7) | 29.1 (4.7) | 29.3 (4.9) | 0.847 |
Note: Values are the mean (standard error of the mean) for 10 subjects who participated in the study in each group, except that the discontinuous variable, Tmax, is given as median (range).
Effect of ARV on DIS
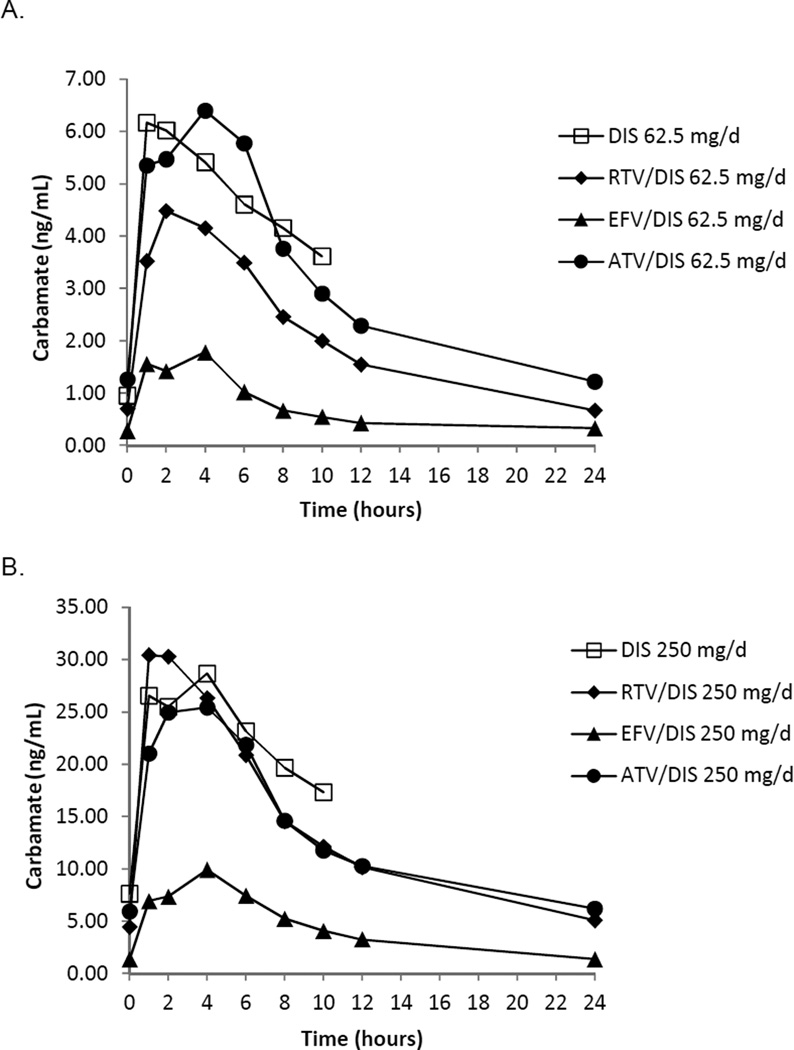
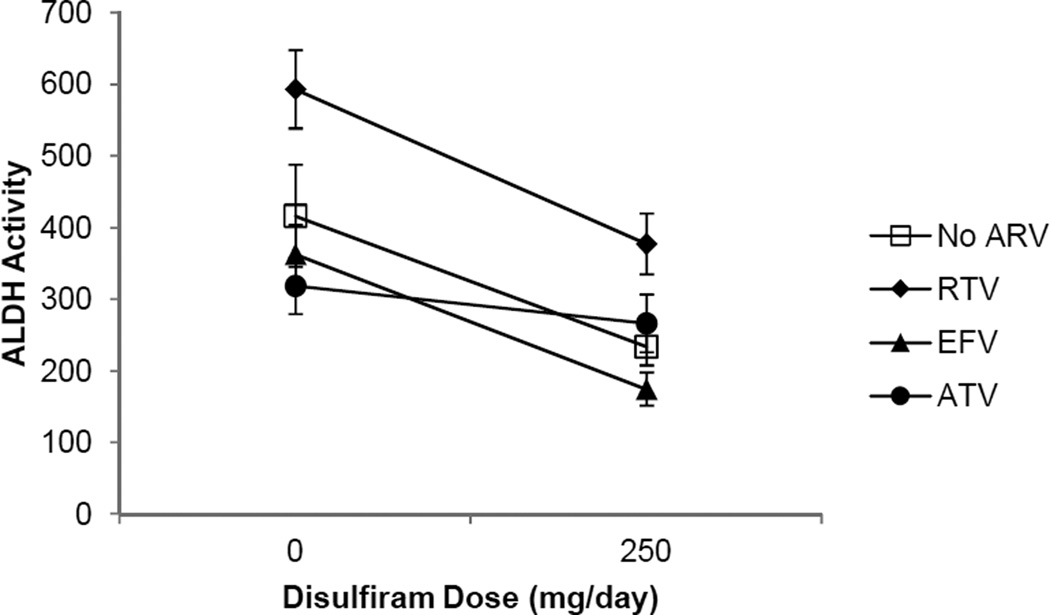
Two indices of DIS activity in the presence of ARV medications were examined with simultaneous, chronic dosing of both the ARV of interest and DIS. The DIS intermediary metabolite, DIS carbamate, is formed following metabolism via the CYP 450 enzyme system and, therefore, is one measure of the effect of ARV medications, which have the potential to alter CYP 450 function, on DIS efficacy. Table 3 shows a dose response for DIS pharmacokinetics parameters as demonstrated by the DIS metabolite, DIS carbamate, for the DIS 62.5 mg daily dose vs. the DIS 250 mg daily dose. A significant effect of ARV administration on pharmacokinetics parameters for DIS 250 mg daily including Cmax, Cmin, and AUC0–10and for DIS 62.5 mg daily Cmax and AUC0–10was observed. The differences were related to significantly lower DIS carbamate Cmax, Cmin, and AUC0–10 with EFV administration (Table 3, Figure 1). The second measure of DIS activity was examination of ALDH activity. ALDH inhibition is the means by which alcohol metabolism is attenuated resulting in a noxious reaction with alcohol consumption when clinical doses (DIS 250 mg daily) are utilized in the treatment of alcohol use disorders. Figure 3 shows reductions in ALDH activity when DIS 250 mg/d is administered alone (p=0.02) and represents a 44% decrease in ALDH activity in this sample. ALDH activity is also significantly decreased when RTV 200 mg/d is administered concurrently with DIS 250 mg/d (p= 0.009) and represents a 36% decrease in ALDH activity. ATV co-administration with DIS 250 mg/d resulted in a 16% reduction in ALDH activity (NS). EFV co-administration with DIS 250 daily resulted in the highest levels of ALDH inhibition at 52% (p=0.002).
Table 3.
Comparative pharmacokinetics for S-Methyl-N-N-diethylthiocarbamate (DIS carbamate) following disulfiram 62.5 mg daily or250 mg daily alone or in combination with antiretroviral medications
| Study Condition |
Tmax (hr) |
Cmax (ng/ml) |
Cmin (ng/ml) |
AUC0-10 (hr*ng/ml) |
|---|---|---|---|---|
| DIS62.5 | 3.8±1.0 | 8.7±1.9 | 0.96±0.2 | 48.4±9.5 |
| EFV+DIS62.5 | 2.5±0.6 | 2.2±0.7* | 0.28±0.1 | 16.9±4.2* |
| RTV+DIS62.5 | 2.6±0.6 | 5.9±0.9 | 0.56±0.30 | 49.7±9.9 |
| ATV+DIS62.5 | 3.1±0.6 | 8.0±1.3 | 0.90±0.3 | 49.6±8.5 |
| p value | 0.558 | 0.004 | 0.271 | 0.020 |
| DIS250 | 3.1±0.6 | 35.6±5.2 | 7.6±1.1 | 230.4±29.4 |
| EFV+DIS250 | 2.4±0.5 | 11.7±2.9* | 1.1±0.4* | 103.1±20.3* |
| RTV+DIS250 | 2.0±0.4 | 39.5±5.4 | 3.9±0.8 | 328.0±41.8 |
| ATV+DIS250 | 2.9±0.5 | 32.1±4.6 | 5.3±1.4 | 200.3±27.9 |
| p value | 0.483 | 0.001 | <0.0001 | 0.001 |
Note: Values are the mean (standard error of the mean) for 10 subjects who participated in the study in each group.
Significantly different from the control
Figure 1.
Effect of Antiretroviral Medications on S-Methyl-N-N-diethylthiocarbamate (DIS Carbamate) at A. Disulfiram 62.5 mg daily or B. Disulfiram 250 mg daily
Discussion
This study shows that DIS can be safely used with commonly prescribed ARV including RTV, ATV, and EFV. DIS has no effect on the pharmacokinetics of any of these medications; therefore ARV dose adjustments when using DIS for treatment of substance use disorders in those receiving these ARV should not be necessary. Two of the ARV studied had significant effects on DIS metabolism. EFV co-administration was associated with significant decreases in DIS carbamate and a moderate increase in ALDH inhibition relative to DIS alone administration. ATV administration with DIS 250 mg daily was associated with a lack of DIS-associated inhibition of ALDH activity. This indicates that DIS at standard clinical doses utilized in the treatment of alcohol use disorders is unlikely to be effective; i.e.: a DIS-alcohol reaction may not occur in an individual receiving an ATV-containing regimen for HIV infection and DIS for an alcohol disorder.
This study was undertaken because the hazardous use of alcohol as well as alcohol use disorders are common in those with HIV infection (9–11), yet pharmacotherapies for alcohol use disorders are infrequently used in the population. This might be largely due to the sparse evidence currently available on the interactions of DIS and ARV. Informing clinicians of the safety of DIS with concurrent use of ARV as well as identifying potential problems in the use of this alcohol pharmacotherapy which has also shown promise in the treatment of cocaine dependence (4) may be helpful in increasing the clinical use of DIS in the HIV-infected population.
Several studies have indicated inhibitory effects of DIS treatment on CYP450 enzymatic activity (e.g. CYP3A4) which are variable (20, 31, 32). A number of ARV are substrates of CYP3A4 including those in this study, EFV (22), ATV (33), and RTV (32). DIS was administered at two doses: 62.5 mg/d and 250 mg/d because these doses have been shown to diminish acute cocaine responses in humans (5). The finding that neither dose had a significant effect on the pharmacokinetics of any of the ARV studied suggests that DIS in clinically relevant doses can be safely used as it pertains to ARV efficacy in HIV-infected people receiving ARV therapy. Of note, the RTV formulation administered in this study was an alcohol-containing gelcap. No participants receiving RTV with DIS experienced symptoms of an alcohol-DIS reaction.
The effect of the ARV on DIS metabolism and ALDH inhibition was consistent with study hypotheses. EFV is known to induce CYP450 enzyme activity; specifically CYP3A4 (21). DIS carbamate, an intermediate in DIS metabolism, is metabolized to S-methyl N,N-diethylthiolcarbamate sulfoxide (DETC-MeSO) by CYP3A4, -2A6, and -2E1 (14) and therefore, it was expected that levels of DIS carbamate would be lower in those receiving EFV as compared to those receiving DIS alone. DETC-MeSO is the DIS metabolite responsible for ALDH inhibition (14). The induction of CYP enzyme activity by EFV would be expected to produce increased DETC-MeSO which would be manifest as a relative increase in the inhibition of ALDH activity. This was observed in that ALDH activity showed the greatest decrease in those receiving EFV and DIS concomitantly with a 52% decline in ALDH activity observed (baseline 363.07 (40.46) mean (SE) vs. following EFV 600 mg/d and DIS 250 mg/d: 174.75 (23.18) (p = 0.002) (shown graphically in Figure 3). As a comparison, baseline ALDH activity in those receiving only DIS 250/d was 416.46 (71.41), which declined to 233.89 (26.14) following DIS 250 mg/d administration (44% decrease from baseline p = 0.020). ATV and RTV were expected to reduce the conversion of the DIS carbamate to DETC-MeSO due to their inhibition of CYP3A4 (23, 34). Thus, it was expected that co-administration of these ARV with DIS would be associated with proportionately less inhibition of ALDH activity relative to disulfiram alone administration. Among subjects receiving RTV 200 mg/d and DIS 250 mg/d, baseline ALDH activity [593.17 (54.6)] significantly declined to 377.28 (42.57) (36% reduction) (p=0.009). A non-significant (16%) decline in ALDH activity from 318.7 (38.91) to 266.36 (39.91) was observed among subjects receiving ATV 400 mg/d with DIS 250 mg/d (shown graphically in Figure 2). It is notable that there was significant variation in individual ALDH activity at baseline; therefore the proportional change in ALDH activity was used as the comparator in this study. Proportional changes in ALDH activity were consistent with the expected effect of the ARV on CYP 450 activity.
Figure 2.
Effect of Disulfiram in Combination with Antiretroviral Medications on ALDH Activity
One difference observed in subjects participating in the ATV and DIS study was an increase in total bilirubin when ATV was administered alone or with DIS. ATV is known to inhibit bilirubin glucuronidation by UDP glucuronosyltransferase 1A1 (UGT 1A1) with a resultant increase in serum bilirubin levels that rapidly reverses on drug discontinuation (35). This hyperbilirubinemia occurs without concomitant increases in transaminases and is not regarded as a sign of liver dysfunction (36).
One of the implications of the effect of ATV on glucuronidation would be in the potential for inhibition of a contributing pathway for metabolism of some DIS metabolites (diethyldithiocarbamate, for example). In humans, glucuronidation has been shown to be responsible for elimination of 1.7% of a single 250 mg dose of DIS while 8.3% of DIS is eliminated by glucuronidation with chronic dosing (37). The inhibition of glucuronidation by co-administration of ATV might increase the amount of DIS metabolite that is subject to CYP 450 metabolism. The inhibition of CYP 3A4 by ATV (34) could result in accumulation of DIS metabolites and a concomitant decrease in the DIS metabolite responsible for inhibition in ALDH (DETC-MeSO). We observed a decrease in ALDH inhibition by DIS when administered with ATV, but we cannot say with certainty the mechanism by which this occurred since we were unable to measure all of the metabolites formed in the DIS metabolic pathway.
A question arising from these results is whether DIS at standard clinical doses used in the treatment of alcohol use disorders (i.e.: 250 mg/d) would be effective as a deterrent to alcohol use in those receiving antiretroviral therapies that contain either EFV, RTV, or ATV. It is not possible to answer this question with precision because no studies have correlated the proportion of reduction in ALDH activity with the occurrence or severity of a DIS-alcohol reaction. However, in the current study there were two participants who consumed alcohol within 3 days of discontinuation of DIS 250 mg/d (this occurred following completion of study procedures). Both reported flushing, and one reported a sensation of labored breathing while the second alcohol consumer stated that mild nausea was the most prominent symptom. Each stated that they had started to experience these symptoms after drinking less than one glass of wine. They were told to immediately discontinue alcohol use and to come for medical evaluation if symptoms worsened. Both reported discontinuation of symptoms within a few hours and neither required medical intervention. ALDH inhibition relative to their baseline ALDH function was determined for each of these individuals and found to be 31% for one subject and 68% for the second participant. While inexact, it would appear that the proportion of ALDH inhibition that occurs in an individual is related to the ability to mount a DIS-alcohol interaction with alcohol consumption while taking DIS. It appears, then, from these results that EFV would not be expected to interfere with DIS-mediated ALDH inhibition, nor were any adverse events associated with the 8% additional ALDH inhibition observed relative to the sample receiving DIS 250 mg/d alone. RTV administration, while associated with a lesser reduction in ALDH inhibition over that observed with DIS alone administration is also not likely to interfere with the occurrence of a DIS-alcohol reaction. ATV administration with DIS 250 mg/d was associated with only a 16% reduction in ALDH activity observed when these medications were administered concomitantly. This may render DIS at standard clinical doses as administered in this study ineffective. Human laboratory studies that include alcohol administration following concomitant administration of clinically relevant doses of the ARV and DIS would be able to conclusively answer the question of DIS efficacy for alcohol dependence in those requiring ART that include these HIV therapeutics.
DIS has also been studied as a treatment for cocaine dependence. The proposed mechanism by which disulfiram alters cocaine responses has been postulated to be related to inhibition of dopamine beta hydroxylase activity which occurs through the disulfiram metabolite, diethyldithiocarbamate (DDTC) (38). Upon oral ingestion, DIS is rapidly reduced to its corresponding thiol, diethyldithiocarbamate (DDTC) (39) and DDTC inactivates dopamine beta hydroxylase by chelation. Because this is the first compound formed in the DIS metabolic cycle; there is no expectation that the effect of ARV on CYP 450 enzyme function would have an effect on this activity. If the hypothesized mechanism for DIS effect on cocaine responses is correct, then DIS would still be expected to be effective for treatment of cocaine use disorders in those receiving ART that contained any of the ARV studied.
There are limitations to this study. The number of ARV studied was limited and sample sizes were relatively small. To address these limitations, ARV selected for study were those expected to be likely to have interactions with DIS based on their known clinical pharmacology, although there are a large number of ARV of potential interest in terms of interactions with DIS. The study design would have benefitted from an assay to detect DETC-MeSO and this was planned at the outset of the study. However, the development of a reliable assay proved challenging leading to the use of DIS carbamate and ALDH activity as surrogate markers of the effects of the ARV studied on DIS.
In summary, the results of drug interaction studies between three ARV that are frequently used in the treatment of HIV infection and DIS are reported. No effect of DIS on ARV pharmacokinetics at standard therapeutic doses of medications was observed, although EFV lowered DIS carbamate plasma concentrations. EFV and RTV did not interfere with the development of significant ALDH inhibition by DIS as was observed with ATV administration, indicating that DIS at standard clinical doses may not be effective in deterring alcohol use if given concomitantly with ATV.
Acknowledgements
The authors thank Vincent Samson, BA for expert technical assistance with this study.
Supported by NIDA NIH grants R01 DA 024982 (EMK) and K24 DA 023359 (EMK).
Footnotes
Conflicts of Interest
E.F. McCance-Katz: none
V.A. Gruber: none
P. Lum: none
G. Beatty: none
Q. Ma: none
R. DiFrancesco: none
J. Hochreiter: none
M.D. Faiman: none
G.D. Morse: none
References
- 1.Wright C, Moore RD. Disulfiram treatment of alcoholism. Am J Med. 1990;88:647–655. doi: 10.1016/0002-9343(90)90534-k. [DOI] [PubMed] [Google Scholar]
- 2.Suh JJ, Pettinati HM, Kampman KM, O’Brien CP. The status of disulfiram: A half a century later. J Clin Psychopharmacol. 2006;26:290–302. doi: 10.1097/01.jcp.0000222512.25649.08. [DOI] [PubMed] [Google Scholar]
- 3.Barth KS, Malcolm RJ. Disulfiram: An old therapeutic with new applications. CNS Neurol Disord Drug Targets. 2010;9:5–12. doi: 10.2174/187152710790966678. [DOI] [PubMed] [Google Scholar]
- 4.Carroll KM, Fenton LR, Ball SA, et al. Efficacy of disulfiram and cognitive behavior therapy in cocaine-dependent outpatients: A randomized, placebo-controlled, clinical trial. Arch Gen Psychiatry. 2004;61:264–272. doi: 10.1001/archpsyc.61.3.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baker JR, Jatlow P, McCance-Katz EF. Disulfiram effects on responses to intravenous cocaine administration. Drug Alcohol Depend. 2007;87:202–209. doi: 10.1016/j.drugalcdep.2006.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Drug-Associated HIV Transmission Continues in the United States. [Accessed October 24, 2012];Centers for Disease Control and Prevention. 2007 http://www.cdc.gov/hiv/resources/factsheets/idu.htm.
- 7.Green TC, Kershaw T, Lin H, et al. Patterns of drug use and abuse among aging adults with and without HIV: A latent class analysis of a US Veteran cohort. Drug Alcohol Depend. 2010;110:208–220. doi: 10.1016/j.drugalcdep.2010.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Metzger DS, Zhang Y. Drug treatment as HIV prevention: Expanding treatment options. Curr HIV/AIDS Rep. 2010;7:220–225. doi: 10.1007/s11904-010-0059-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bonacini M. Alcohol use among patients with HIV infection. Ann Hepatol. 2011;10:502–507. [PubMed] [Google Scholar]
- 10.Samet JH, Horton NJ, Meli S, Freedberg KA, Palepu A. Alcohol consumption and antiretroviral adherence among HIV-infected persons with alcohol problems. Alcohol Clin Exp Res. 2004;28:572–577. doi: 10.1097/01.alc.0000122103.74491.78. [DOI] [PubMed] [Google Scholar]
- 11.Braithwaite RS, Conigliaro J, Roberts MS, et al. Estimating the impact of alcohol consumption on survival for HIV+ individuals. AIDS Care. 2007;19:459–466. doi: 10.1080/09540120601095734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Madan A, Faiman MD. Characterization of diethyldithiocarbamate methyl ester sulfine as an intermediate in the bioactivation of disulfiram. J Pharmacol Exp Ther. 1995;272:775–780. [PubMed] [Google Scholar]
- 13.Madan A, Parkinson A, Faiman MD. Role of flavin-dependent monooxygenases and cytochrome P450 enzymes in the sulfoxidation of S-methyl N,N-diethylthiolcarbamate. Biochem Pharmacol. 1993;46:2291–2297. doi: 10.1016/0006-2952(93)90620-c. [DOI] [PubMed] [Google Scholar]
- 14.Madan A, Parkinson A, Faiman MD. Identification of the human and rat P450 enzymes responsible for the sulfoxidation of S-methyl N,N-diethylthiolcarbamate (DETC-ME). The terminal step in the bioactivation of disulfiram. Drug Metab Dispos. 1995;23:1153–1162. [PubMed] [Google Scholar]
- 15.Madan A, Parkinson A, Faiman MD. Identification of the human P-450 enzymes responsible for the sulfoxidation and thiono-oxidation of diethyldithiocarbamate methyl ester: role of P-450 enzymes in disulfiram bioactivation. Alcohol Clin Exp Res. 1998;22:1212–1219. [PubMed] [Google Scholar]
- 16.Kharasch ED, Hankins DC, Jubert C, Thummel KE, Taraday JK. Lack of single-dose disulfiram effects on cytochrome P-450 2C9, 2C19, 2D6, and 3A4 activities: Evidence for specificity toward P-450 2E1. Drug Metab Dispos. 1999;27:717–723. [PubMed] [Google Scholar]
- 17.Loi CM, Day JD, Jue SG, et al. Dose-dependent inhibition of theophylline metabolism by disulfiram in recovering alcoholics. Clin Pharmacol Ther. 1989;45:476–486. doi: 10.1038/clpt.1989.61. [DOI] [PubMed] [Google Scholar]
- 18.Enghusen Poulsen H, Loft S, Andersen JR, Andersen M. Disulfiram therapy: Adverse drug interactions and interactions. Acta Psychiatr Scand Suppl. 1992;369:359–366. doi: 10.1111/j.1600-0447.1992.tb03317.x. [DOI] [PubMed] [Google Scholar]
- 19.Poulsen HE, Ranek L, Jorgensen L. The influence of disulfiram on acetaminophen metabolism in man. Xenobiotica. 1991;21:243–249. doi: 10.3109/00498259109039466. [DOI] [PubMed] [Google Scholar]
- 20.Honjo T, Netter KJ. Inhibition of drug demethylation by disulfiram in vivo and in vitro. Biochem Pharmacol. 1969;18:2681–2683. doi: 10.1016/0006-2952(69)90201-9. [DOI] [PubMed] [Google Scholar]
- 21.Hariparsad N, Nallani SC, Sane RS, Buckley DJ, Buckley AR, Desai PB. Induction of CYP3A4 by efavirenz in primary human hepatocytes: Comparison with rifampin and phenobarbital. J Clin Pharmacol. 2004;44:1273–1281. doi: 10.1177/0091270004269142. [DOI] [PubMed] [Google Scholar]
- 22. [Accessed November 1, 2012];Sustiva (efavirenz) capsules and tablets package insert. http://www.accessdata.fda.gov/drugsatfda_docs/label/2011/020972s038lbl.pdf.
- 23.Kumar GN, Rodrigues AD, Buko AM, Denissen JF. Cytochrome P450-mediated metabolism of the HIV-1 protease inhibitor ritonavir (ABT-538) in human liver microsomes. J Pharmacol Exp Ther. 1996;277:423–431. [PubMed] [Google Scholar]
- 24.Sheehan DV, Lecrubier Y, Sheehan HK, et al. The mini-international neuropsychiatric interview (M.I.N.I): The development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 1998;59(Suppl 20):22–33. [PubMed] [Google Scholar]
- 25.McCance-Katz EF, Moody DE, Morse GD, et al. Interactions between buprenorphine and antiretrovirals. I. Nonnucleoside reverse-transcriptase inhibitors efavirenz and delavirdine. Clin Infect Dis. 2006;43(Suppl 4):S224–S234. doi: 10.1086/508187. [DOI] [PubMed] [Google Scholar]
- 26.Povsic TJ, Zavodni KL, Kelly FL, et al. Circulating progenitor cells can be reliably identified on the basis of aldehyde dehydrogenase activity. J Am Coll Cardiol. 2007;50:2243–2248. doi: 10.1016/j.jacc.2007.08.033. [DOI] [PubMed] [Google Scholar]
- 27.Hochreiter J, McCance-Katz EF, Lapham J, Ma Q, Morse GD. Disulfiram metabolite S-methyl-N,N-diethylthiocarbamate quantitation in human plasma with reverse phase ultra performance liquid chromatography and mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci. 2012;897:80–84. doi: 10.1016/j.jchromb.2012.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Keil K, Frerichs VA, DiFrancesco R, Morse GD. Reverse phase high performance liquid chromatography method for the analysis of amprenavir, efavirenz, indinavir, lopinavir, nelfinavir and its active metabolite (M8), ritonavir, and saquinavir in heparinized human plasma. Ther Drug Monit. 2003;25:340–346. doi: 10.1097/00007691-200306000-00015. [DOI] [PubMed] [Google Scholar]
- 29.Keil K, Hochreiter J, DiFrancesco R, et al. Integration of atazanavir into an existing liquid chromatography UV method for protease inhibitors: validation and application. Ther Drug Monit. 2007;29:103–109. doi: 10.1097/FTD.0b013e3180318ef3. [DOI] [PubMed] [Google Scholar]
- 30.Xing S, Bullen CK, Shroff NS, et al. Disulfiram reactivates latent HIV-1 in a Bcl-2-transduced primary CD4+ T cell model without inducing global T cell activation. J Virol. 2011;85:6060–6064. doi: 10.1128/JVI.02033-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Peachey JE, Brien JF, Roach CA, Loomis CW. A comparative review of the pharmacological and toxicological properties of disulfiram and calcium carbimide. J Clin Psychopharmacol. 1981;1:21–26. doi: 10.1097/00004714-198101000-00005. [DOI] [PubMed] [Google Scholar]
- 32.Freundt KJ. Variable inhibition of human hepatic drug metabolizing enzymes by disulfiram. Int J Clin Pharmacol Biopharm. 1978;16:323–330. [PubMed] [Google Scholar]
- 33. [Accessed November 11, 2012];Reyataz (atazanavir sulfate) capsules package insert. Available at: http://packageinserts.bms.com/pi/pi_reyataz.pdf.
- 34.Perloff ES, Duan SX, Skolnik PR, Greenblatt DJ, von Moltke LL. Atazanavir: Effects on P-glycoprotein transport and CYP3A metabolism in vitro. Drug Metab Dispos. 2005;33:764–770. doi: 10.1124/dmd.104.002931. [DOI] [PubMed] [Google Scholar]
- 35.Zhang D, Chando TJ, Everett DW, Patten CJ, Dehal SS, Humphreys WG. In vitro inhibition of UDP glucuronosyltransferases by atazanavir and other HIV protease inhibitors and the relationship of this property to in vivo bilirubin glucuronidation. Drug Metab Dispos. 2005;33:1729–1739. doi: 10.1124/dmd.105.005447. [DOI] [PubMed] [Google Scholar]
- 36.Bristol Myers Squibb. Reyataz (atazanavir) Prescribing information. Physicians’ Desk Reference. 2009:3299–3310. [Google Scholar]
- 37.Faiman MD, Jensen JC, Lacoursiere RB. Elimination kinetics of disulfiram in alcoholics after single and repeated doses. Clin Pharmacol Ther. 1984;36(4):520–526. doi: 10.1038/clpt.1984.213. [DOI] [PubMed] [Google Scholar]
- 38.Baker JR, Jatlow P, McCance-Katz EF. Disulfiram effects on responses to intravenous cocaine administration. Drug Alcohol Depend. 2000;87:202–209. doi: 10.1016/j.drugalcdep.2006.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bode H. Systematic studies on the application of diethyl dithiocarbamate in analysis. Chem. 1954;142:414. [Google Scholar]