Abstract
Background
It is not well established how psychosocial factors like social support and depression affect health-related quality of life in multimorbid and elderly patients. We investigated whether depressive mood mediates the influence of social support on health-related quality of life.
Methods
Cross-sectional data of 3,189 multimorbid patients from the baseline assessment of the German MultiCare cohort study were used. Mediation was tested using the approach described by Baron and Kenny based on multiple linear regression, and controlling for socioeconomic variables and burden of multimorbidity.
Results
Mediation analyses confirmed that depressive mood mediates the influence of social support on health-related quality of life (Sobel’s p < 0.001). Multiple linear regression showed that the influence of depressive mood (β = −0.341, p < 0.01) on health-related quality of life is greater than the influence of multimorbidity (β = −0.234, p < 0.01).
Conclusion
Social support influences health-related quality of life, but this association is strongly mediated by depressive mood. Depression should be taken into consideration in research on multimorbidity, and clinicians should be aware of its importance when caring for multimorbid patients.
Trial registration
Keywords: Multimorbidity, Chronic medical conditions, Coping, Primary care, Family practice, Social support, Health-related quality of life, Depression, Elderly patients
Background
Health-related quality of life is a measure of subjective health that complements disease-specific outcomes in multimorbid patients, because good quality of life is of value in itself and it is an independent predictor of mortality [1,2]. All relevant factors that might affect health-related quality of life in multimorbid patients need to be looked at in order to gain a more detailed biopsychosocial understanding [3] of multimorbidity. These include biological factors such as the extent of multimorbidity, as well as psychosocial factors such as social support and depression. The present study aims to clarify the relationship between quality of life, depression and social support in multimorbid patients.
Multimorbidity is commonly defined as the co-occurrence of two or more diseases and medical conditions within one person [4]. As the number of simultaneous chronic diseases increases with age, multimorbidity is common in elderly patients [5,6]. In a recent study the prevalence of multimorbidity in primary care practices was 65% in elderly persons [5]. Previous research has attempted to increase our understanding of multimorbidity by identifying patterns of disease combinations [7-9]. Diseases tend to co-occur when they share common risk factors or pathophysiological pathways [10], or, given the high prevalence of many diseases in the elderly, by coincidence. Based on factor-analysis, Schäfer et al. [9] identified three multimorbidity patterns: cardiovascular/metabolic disorders (CMD), anxiety/depression/somatoform disorders and pain (ADS/P) and neuropsychiatric disorders (NPS).
Multimorbidity has several adverse consequences for patients, e.g. polypharmacy and decreases in functional abilities. Furthermore, it is known that the burden of multimorbidity consistently leads to impaired health-related quality of life in primary care patients [11,12].
Social support by relatives, friends or professionals can promote adaptation to and coping with chronic illness and multimorbidity [13,14]. Social support is a broad concept that is commonly divided into instrumental and emotional support, as well as into actually provided, received, and perceived social support [15]. Perceived social support can be defined as ‘the perception or experience that one is loved and cared for by others, esteemed and valued, and part of a social network of mutual assistance and obligations’ (Wills cited in [15]). Social support is positively correlated with health-related quality of life [16,17] and was identified by Fortin et al. [11] to be one of the most important factors predicting health-related quality of life in multimorbid patients. Therefore, understanding how social support and quality of life are related, can inform primary care interventions addressing multimorbid patients.
Social support is negatively correlated with depression [18,19]. Impaired social support and feelings of loneliness are considered to be risk factors for depression in the elderly [20,21]. As social support is associated both with health-related quality of life and with depression, the question arises whether and how these factors interact.
Psychological distress [22] and depression [23,24] are known to affect health-related quality of life. This association is especially important in multimorbid patients because duration and severity of depression have a higher negative impact on health-related quality of life than physical chronic conditions [25]. Additionally, depression is a common [26] and often chronic [27] comorbidity in elderly patients. And, in primary care patients, the probability of suffering from depression grows with increasing physical morbidity [28].
In previous research, depression was shown to be a mediating variable in the relationship between social support and health-related quality of life in patients with HIV/AIDS [18,29]. As an explanation, Bekele et al. have proposed that either a perceived lack of social support increases perceived threats of stressful events, or a high level of perceived social support decreases perceived threats of stressful events [29]. This, in turn, leads to either an increase or decrease in depressive symptoms, and influences health-related quality of life accordingly. As social support has a positive and depression a detrimental effect on health-related quality of life in multimorbid patients, a similar relationship to that observed in patients with HIV/AIDS may exist.
To clarify the direct and indirect effects of social support and depressive mood on health-related quality of life in multimorbid patients, we investigated the hypothesis that depressive mood mediates the influence of social support on health-related quality of life in these patients. Additionally, we investigated whether the hypothesis holds true for the three different multimorbidity patterns. The analytic relation is depicted in Figure 1.
Figure 1.
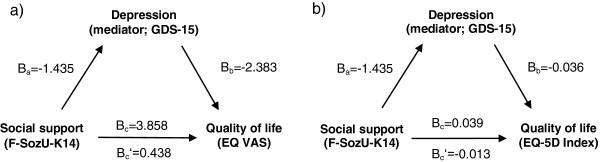
Analytic relation between study variables. Legend: Analytical relation between social support (F-SozU-K14), depressive mood (GDS-15) and health-related quality of life (outcome variable EQ VAS (a) and EQ-5D Index (b)). Values are unstandardized regression coefficients.
Methods
Cross-sectional data come from the baseline assessment of the German MultiCare study, a longitudinal, prospective observational cohort study of multimorbid elderly patients [30]. 3,189 patients were recruited from 158 general practices in eight study centres across Germany. The study protocol was approved by the Ethics Committee of the Medical Association of Hamburg.
Participants and sampling
Included patients were between 65 and 85 years of age, had visited their general practitioner (GP) at least once within the previous three-month period and had at least three chronic medical conditions from a list of 29 common diseases. Patients were randomly selected based on medical records from primary care practices but were excluded if they were unable to participate in interviews (deaf, blind or unable to speak German), if they were not regular patients of the respective practice, if they were living in nursing homes, if they were not able to give informed consent (e.g. demented patients), or if they had an acute illness which was expected to result in death within three months. The complete list of diseases and further details on the study design can be found elsewhere [30,31]. Data were obtained from GPs’ medical records and from standardized comprehensive interviews with patients.
Measures
Depressive mood
We used the Geriatric Depression Scale, which was developed for assessment of depression in elderly persons [32]. It avoids assessment of physical symptoms, which in elderly and comorbid patients cannot clearly be attributed to depression [33]. Validation studies of the Geriatric Depression Scale in hospitals and nursing-home residents showed good results [34]. In primary care populations, it appears to be preferable to use the short version of the Geriatric Depression Scale (GDS-15) instead of the long version [35], and hence the short version was used in this study. The German version of the GDS-15, used here, showed good psychometric properties [36]. The scale comprises 15 items that can be answered either with yes or no, with a threshold score of ≥6 out of 15 making major depression likely [36]. As others have done before, we used the GDS-15 as a continuous scale in our mediator analysis to assess for depressive mood [24], based on the assumption that a higher score on the GDS-15 reflects greater depressive mood than a lower score, regardless of the threshold.
Social support
To assess perceived social support, the short form of the Social Support Questionnaire was used (F-SozU-K14). The F-SozU-K14 is commonly used in Germany (e.g. [37]) and good evidence for its validity exists [38]. A continuous summary score is calculated from its 14 items, with higher values indicating more perceived social support. The F-SozU-K14 assesses perceived emotional support, perceived practical support and perceived social integration. However, for the short form of the Social Support Questionnaire, no differentiation to these subscales is recommended by the authors [39], which is why the summary score was used.
Health-related quality of life
Health-related quality of life was measured using the EuroQol-5D-3L (EQ-5D) instrument [40]. Patients were asked to self-rate their current health state on a visual analogue scale (EQ VAS) from 0 to 100. Additionally, the EQ-5D assesses five dimensions of the current health state of patients: mobility, self-care, usual activities, pain/discomfort, and anxiety/depression. Each dimension is assessed on three levels: no problems, some problems, or severe problems. Thereby a total of 243 possible health states results, from which a single continuous index score can be obtained (EQ-5D Index; 1 represents perfect health and 0 represents death). This is carried out using health-state valuation data from valuation studies in general populations. We used the European valuation data based on Greiner et al. [41] reported in Szende et al. [42]. In our analyses we used both the EQ VAS and the EQ-5D Index variables to assess the outcome of health-related quality of life.
The EQ-5D is among the briefest health index measures and due to its ease of application has high completion rates in elderly populations, but it has been criticized for being less sensitive to change than the SF-36 [43]. In a multimorbid patient sample, however, its lower sensitivity is probably less relevant, because patients are relatively sick and inter-individual differences more pronounced [34]. We therefore consider the EQ-5D to be a reliable and valid instrument for a multimorbid patient sample.
Control variables
Socioeconomic control variables used were: age, gender, educational level, income and living-situation. Educational level was divided into three categories, based on the CASMIN-classification [44]: 1) inadequately completed general education, general elementary education or basic vocational qualification; 2) intermediate qualification or general maturity certificate; 3) lower or higher tertiary education. Income was reported as household-size adjusted net income per month. Participants were classified as either ‘living with a partner or relative’, or as ‘living alone’. ‘Living alone’ included assisted living or living in retirement homes.
As a control variable for the disease burden of multimorbidity, a weighted disease count was included in the model. There is no consensus on how to measure multimorbidity and many different measures exist [45]. Because a multimorbidity measure incorporating severity of disease was described to be associated with psychological distress, while a simple disease count was not [22], it seemed appropriate to account for disease severity. In this study, patients’ diagnoses and severity of diseases were assessed in interviews with GPs. The weighted disease count was then calculated by summing up the severity ratings (‘marginal’ = 0, ‘low’ = 1, ‘medium’ = 2, ‘severe’ = 3 and ‘very severe’ = 4) given by the physician. Pearson’s correlation of the weighted and unweighted disease count was r = 0.774 (p < 0.01).
To test the mediation hypothesis for different multimorbidity patterns, patients were assigned to the three patterns described above (CMD, ADS/P and NPS) if they had at least three diseases belonging to one of these groups, as described by Schäfer et al. [9].
Missing values
Missing data were imputed using the hot deck method from donors, and identified on the basis of the nearest Gower distance. 2,720 patients (85.3%) had no missing values and were eligible as donors. Eight participants with missing data needed for the calculation of the EQ-5D Index variable were excluded from further analysis. A more detailed description of the imputation process can be found elsewhere [31].
Statistical analyses
A correlation matrix was calculated using Pearson’s coefficients for continuous variables or Spearman’s coefficients for nominal and ordinal variables. To assess both direct and indirect effects of social support on health-related quality of life, we tested the mediation hypothesis as described by Baron and Kenny [46]. This approach is a measurement-of-mediation design. It is used to statistically measure the mediator variable’s effect, in contrast to experimental approaches like the experimental-chain-design, where the mediator variable is directly manipulated [47]. We used multiple linear regression to calculate unstandardized and standardized coefficients of variables as well as adjusted R-squares (R2). We confirmed assumptions for regression analyses by checking for linearity between variables based on plotting and curve-fitting procedures, by excluding multicollinearity based on variance inflation factors, and by assessing normal distribution of residuals graphically. Control variables were used in all regression calculations. Testing mediation requires three regression models, in which the following conditions must be fulfilled: first, the predictor variable (social support) must significantly influence the mediator variable (depressive mood); second, the predictor variable must significantly influence the outcome variable (health-related quality of life); and third, the predictor variable’s influence on the outcome variable must be greatly reduced or become non-significant when the mediator variable is included in the model. Mediation analyses were done separately for the two outcome variables EQ VAS and EQ-5D Index. To test significance of the indirect path via the mediator variable, we used Sobel’s test applying a utility provided by Preacher and Leonardelli [48]. To investigate whether the mediation hypothesis holds true in different multimorbidity patterns, we also conducted the mediation analyses separately for all patients exclusively assigned to the CMD pattern and for all patients exclusively assigned to the ADS/P pattern. Based on the assumption that differences would be more pronounced in patients that were assigned to one pattern alone, all patients assigned to multiple patterns were excluded. The NPS pattern was not accounted for, as only four patients were exclusively assigned to it. All resulting values were said to be significant at a level of p < 0.05. Analyses were done using SPSS version 19.0.
Results
Sample characteristics
The total sample at baseline consisted of 3,189 patients. The mean age was 74.4 years and 59.3% of patients were female (see Table 1). 62.3% of patients had a low educational status. The mean number of chronic conditions was 7.0. The three most common diagnoses were hypertension, disorders of lipid metabolism and chronic low back pain. A more detailed description of age, gender, and socio-economic characteristics of the study cohort can be found elsewhere [31].
Table 1.
Characteristics of the study population and multimorbidity patterns
| All (n = 3,189) | CMD (n = 937) | ADS/P (n = 748) | |
|---|---|---|---|
| Age: mean (SD) |
74.4 (5.2) years |
74.3 (5.2) years |
73.8 (5.2) years |
| Gender: N (%) female |
1,891 (59.3) |
362 (38.6) |
607 (81.1) |
| Living situation: N (%) |
|
|
|
| With partner or family member |
2,000 (62.7) |
635 (67.8) |
424 (56.7) |
| Alone, assisted living or retirement home |
1,189 (37.3) |
302 (32.2) |
324 (43.3) |
| Education: N (%) |
|
|
|
| Low |
1,986 (62.3) |
591 (63.1) |
439 (58.7) |
| Medium |
856 (26.8) |
227 (24.2) |
244 (32.6) |
| High |
347 (10.9) |
119 (12.7) |
65 (8.7) |
| Income: mean (SD) |
1,412 (704) Euros |
1,433 (838) Euros |
1,404 (596) Euros |
| Number of chronic conditions (SD) |
7.0 (2.5) |
6.2 (1.5) |
6.2 (1.6) |
| Prevalence of most common conditions |
|
|
|
| Hypertension |
77.9% |
90.0% |
54.4% |
| Disorders of lipid metabolism |
58.5% |
68.5% |
36.6% |
| Chronic low back pain |
49.5% |
24.3% |
62.7% |
| Depression |
|
|
|
| Prevalence |
17.7% |
6.1% |
25.4% |
| Mean GDS-15 (SD) |
2.57 (2.60) |
2.35 (2.36) |
2.45 (2.60) |
| Perceived Social Support |
|
|
|
| F-SozU-K14: mean (SD) |
4.1 (0.69) |
4.11 (0.66) |
4.14 (0.68) |
| Health-related quality of life |
|
|
|
| EQ VAS: mean (SD) |
62.4 (18.2) |
64 (18) |
64 (18) |
| EQ-5D Index: mean (SD) | 0.70 (0.23) | 0.75 (0.22) | 0.70 (0.22) |
SD: Standard deviation.
CMD: cardiovascular/metabolic disorders pattern.
ADS/P: anxiety/depression/somatoform disorders and pain pattern.
In the analysis of multimorbidity patterns, 937 patients were exclusively assigned to the CMD pattern and 748 patients to the ADS/P pattern. Further characteristics on the subsamples are displayed in Table 1.
Based on the GDS-15, 401 patients were likely to have depression. Of those, 144 (36%) had previously been diagnosed with depression, according to GP records. Patients that were likely to have depression consistently reported more problems in all five dimensions of health-related quality of life than patients unlikely to have depression.
Correlations
We examined correlations among variables for magnitude and plausibility with regard to our hypothesis (Table 2). Social support correlated negatively with burden of multimorbidity and depressive mood, and positively with both measures of health-related quality of life (EQ VAS and EQ-5D Index). Health-related quality of life, as measured either by the EQ VAS or the EQ-5D Index, correlated negatively with the weighted disease count and with depressive mood.
Table 2.
Correlations and descriptive statistics of study variables
| EQ VAS | EQ-5D Index | GDS-15 | F-SozU-K14 | Weighted disease count | Age | Gender | Educational level | Household income | Living with partner | Mean | Standard deviation | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| EQ VAS |
- |
|
|
|
|
|
|
|
|
|
62.4 |
18.2 |
| EQ-5D Index |
.530** |
- |
|
|
|
|
|
|
|
|
0.703 |
0.23 |
| GDS-15 |
-.406** |
-.446** |
- |
|
|
|
|
|
|
|
2.57 |
2.60 |
| F-SozU-K14 |
.178** |
.140** |
-.407** |
- |
|
|
|
|
|
|
4.01 |
0.69 |
| Weighted disease count |
-.315** |
-.295** |
.197** |
-.076** |
- |
|
|
|
|
|
11.26 |
5.14 |
| Age |
-.112** |
-.108** |
.125** |
-.121** |
.162** |
- |
|
|
|
|
74.4 |
5.19 |
| Gender (female = 1)† |
-.060** |
-.164** |
.092** |
.005 |
-.042* |
.054** |
- |
|
|
|
- |
- |
| Educational level† |
.108** |
.103** |
-.068** |
.016 |
-.117** |
-.054** |
-.111** |
- |
|
|
- |
- |
| Household income |
.127** |
.086** |
-.139** |
.072** |
-.076** |
-.007 |
-.123** |
.258** |
- |
|
1,412 |
706 |
| Living with partner† | .062** | .070** | -.117** | .128** | -.028 | -.191** | -.314** | .066** | .046** | - | - | - |
**Correlation is significant at p < 0.01 (two-sided).
*Correlation is significant at p < 0.05 (two-sided).
†Spearman’s correlation coefficient. All others are Pearson’s coefficients.
Mediator analyses
The predictor variable (social support) significantly influenced the proposed mediator (depressive mood) in a linear regression model; this first model is the same for both outcome variables. The unstandardized regression coefficient Ba was −1.435 (p < 0.001, standard error SE = 0.061). The coefficients used for testing mediation are also shown in Figure 1.
Mediator analysis with EQ VAS
Our first analysis used EQ VAS as outcome variable. The predictor variable social support significantly influenced the outcome variable (step 1 in Table 3); the unstandardized coefficient Bc was 3.858 (p < 0.001, SE = 0.446). When adding the proposed mediator (depressive mood) to the linear regression model (step 2 in Table 3), the social support coefficient decreased to Bc’ = 0.438 (SE = 0.457) and lost statistical significance (p = 0.338), suggesting perfect mediation according to Baron and Kenny. Depressive mood significantly influenced the outcome variable with a coefficient Bb of −2.383 (p < 0.001, SE = 0.123). The indirect pathway was significant at p < 0.001, based on Sobel’s test.
Table 3.
Effects of social support and depression on health-related quality of life estimated using linear regression
| |
EQ VAS (N = 3,189) |
EQ-5D Index (N = 3,181
†
) |
||||||
|---|---|---|---|---|---|---|---|---|
|
Step 1 |
|
Step 2 |
|
Step 1 |
|
Step 2 |
|
|
| Predictors | B [95%-CI] | β | B [95%-CI] | β | B [95%-CI] | β | B [95%-CI] | β |
| Social support |
3.858** [2.983;4.733] |
0.146** |
0.438 [−0.458;1.334] |
0.017 |
0.039** [0.028;0.050] |
0.116** |
−0.013* [−0.024;-0.002] |
−0.038* |
| Depressive mood |
- |
- |
−2.383** [−2.632;-2.143] |
−0.341** |
- |
- |
−0.036** [−0.039;-0.033] |
−0.409** |
| Control variables |
|
|
|
|
|
|
|
|
| Multimorbidity |
−1.022** [−1.139;-0.904] |
−0.288** |
−0.830** [−0.942;-0.718] |
−0.234** |
−0.013** [−0.014;-0.011] |
−0.281** |
−0.010** [−0.011;-0.008] |
−0.216** |
| Age |
−0.149* [−0.267;-0.032] |
−0.043* |
−0.096 [−0.207;0.015] |
−0.027 |
−0.002* [−0.003;0.000] |
−0.042* |
−0.001 [−0.002;0.000] |
−0.024 |
| Gender |
−1.797* [−3.078;-0.516] |
−0.049* |
−0.846 [−2.061;0.369] |
−0.023 |
−0.077** [−0.093;-0.061] |
−0.165** |
−0.063** [−0.078;-0.048] |
−0.134** |
| Educational level |
1.218* [0.317;2.120] |
0.046* |
1.424** [0.572;2.277] |
0.054** |
0.013* [−0.002;0.025] |
0.040* |
0.016** [0.006;0.027] |
0.049** |
| Income |
0.002** [0.001;0.003] |
0.077** |
0.001** [0.000;0.002] |
0.044** |
0.000 [0.000;0.000] |
0.026 |
0.000 [0.000;0.000] |
−0.013 |
| Living with partner |
−0.170 [−1.491;1.151] |
−0.005 |
−0.520 [−1.769;0.729] |
−0.014 |
−0.014 [0.030;0.003] |
−0.028 |
−0.019* [−0.035;-0.004] |
−0.040* |
| R2 (adjusted) | 0.137** | 0.228** | 0.132** | 0.264** | ||||
**significant at p < 0.01.
*significant at p < 0.05.
†EQ-5D data was missing for eight participants and these data were not imputed.
B: unstandardized regression coefficient (95%-CI: 95%-confidence interval).
β: standardized regression coefficient.
Mediator analysis with EQ-5D Index
The same analysis was done using the EQ-5D Index as outcome variable. Again, the predictor variable significantly influenced health-related quality of life (step 1 in Table 3). After adding depressive mood to the model, the unstandardized coefficient decreased from Bc = 0.039 (p < 0.001, SE = 0.006) to Bc’ = −0.013 (95%-CI: −0.024 to −0.002, SE = 0.006) and remained significant at p = 0.023 (step 2 in Table 3). Depressive mood still exerted significant influence on the EQ-5D Index variable with a coefficient Bb = −0.036 (p < 0.001, SE = 0.002). The indirect path was significant at p < 0.001, according to Sobel’s test.
Assessment of overall strength of our regression model showed that the addition of the mediator variable depressive mood to the model greatly increased R2 on both occasions: when using EQ VAS as outcome variable, R2 increased from 0.137 to 0.228 (Table 3) and when using EQ-5D Index, R2 increased from 0.132 to 0.264.
Further variables
The weighted disease count as a measure of multimorbidity and level of education were the only other variables with a significant influence on health-related quality of life in both models after inclusion of depressive mood. Comparison of standardized regression coefficients showed health-related quality of life to be more strongly affected by depressive mood than by the weighted disease count (Table 3). The influence of level of education was marginal.
Multimorbidity patterns
When conducting the mediation analysis in the multimorbidity patterns of CMD and ADS/P, results differed only marginally between the two groups and from the overall analysis. As no meaningful difference between patients in the various patterns was identified, results are not shown.
Discussion
Key findings
Our results show that depressive mood mediates the association between social support and health-related quality of life in multimorbid, elderly patients. This finding holds true in the overall sample and in the two groups of distinct multimorbidity patterns (CMD and ADSP). The fact that multimorbidity patterns did not differ significantly in our analysis suggests that no disease-specific mechanisms are at work, at least with regard to social support and health-related quality of life. In view of this, it may make more sense to consider general disease susceptibility, related to psychosocial factors such as coping style, as a possible explanation of the results [49]. In our analyses, all main criteria for mediation were fulfilled, including significance of the indirect path. Interestingly, the social support coefficient changed from positive to negative in the EQ-5D Index analysis, which may be the result of a suppressor effect. As it decreased markedly after accounting for depressive mood, and confidence intervals were close to zero, we interpreted this result to be in agreement with the mediation hypothesis.
In both analyses, the large increase in R2-values after accounting for depressive mood suggests that although the total effect of social support is mediated by depressive mood, the total effect of depressive mood cannot be explained through social support alone. Therefore additional unaccounted variables are of importance when predicting depressive mood in multimorbid patients.
In addition, our data showed that depressive mood affects health-related quality of life more than the overall disease burden of multimorbidity (as measured by the weighted disease count), which agrees with previous research [25]. For comparison with previous research it should be borne in mind that we have considered depression in late life to be a continuous concept. We therefore used a continuous score and did not dichotomize the GDS-15.
For a theoretical explanation of our results, we draw on cognitive appraisal theory, which is commonly applied in research on adaptation to chronic diseases [13,50]. According to this theory, a person’s encounter with stress leads to a primary appraisal of stressors, in this case multiple illnesses, and to a secondary appraisal of coping resources. An adverse primary appraisal of threat and harm resulting from multiple chronic diseases compounded by a perceived lack of social support in the secondary appraisal process could result in depressive mood. Conversely, the perception of good social support could balance a harmful primary appraisal. The level of depressive mood then influences health-related quality of life either positively or negatively. Cognitive appraisal takes part in coping with chronic diseases and knowledge of the processes involved can aid GPs when supporting patients [50].
To our knowledge, depression as a mediator between social support and health-related quality of life has previously only been studied in patients with HIV/AIDS: in the study by Jia et al. [18] the effect of social support on physical and mental health-related quality of life was completely mediated by depression. In contrast, in the study by Bekele et al. [29] mediation was not complete and a significant, yet small, direct effect of social support on health-related quality of life remained after accounting for depression. Sociodemographic and medical differences between the samples of HIV/AIDS patients and our sample limit direct comparison, but we believe that the similar findings in all samples support the hypothesis.
Strengths and limitations
Major strengths of our study are its large sample size and its coverage of many different diseases. We consider our sample of 3,189 patients with a multitude of common diseases to be highly representative of elderly, multimorbid patients in primary care settings and would contend that our results are more suited to be generalized than studies limited to a single chronic disease. This is of great advantage especially for primary care practice.
Our study is limited by its cross-sectional design (longitudinal results will, however, be available from the MultiCare study in the future). Consequently, one criterion for mediation mentioned by Baron and Kenny [46] cannot be checked: namely that the mediator is not caused by the outcome variable. We cannot exclude this possibility as there is no way of knowing the sequence of events in our sample. Here, experimental designs to test mediation could provide stronger evidence of causal relations among variables [47]. We used a unique multimorbidity score, as described in detail in the methods section, which we consider to be superior to a simple disease count for this study. However, it limits comparison to other studies on multimorbidity. Given the multitude of multimorbidity scores [45], this is a common limitation of multimorbidity research.
Implications for research
Future research needs to clarify and integrate further variables in a model of social support and health-related quality of life. Several other variables have been shown to act as mediators between social support and health-related quality of life: sense of coherence [51], self-esteem and control beliefs [52]. As shown by Schwarzer et al. [53], self-efficacy can act as an intermediate variable between social support and depression. Furthermore, personality traits such as neuroticism may affect all relevant variables: social support, depressive mood and self-ratings of quality of life [54,55]. Altogether this points to the complexity of what are often reciprocal relations. Ideally this should lead to integrated models that include all relevant variables and are tested in longitudinal rather than cross-sectional studies. The next step would be the design of clinical studies modifying one or more of the implicated variables in the attempt to improve quality of life of multimorbid patients in primary care.
Implications for family practice
Our data add to existing evidence showing the importance of depression as comorbidity in patients with physical illness [23]. The relative importance of depressive mood compared to social support in our study’s results suggests that, regarding health-related quality of life, interventions directed at depressive mood are probably more effective than interventions to improve social support. As Löffler et al. showed, coping with multimorbidity is an active process by patients, often requiring the utilisation of all their resources including social support [14]. Reduced energy and decreased activity as hallmark symptoms of depression potentially limit the coping process with multimorbidity and therefore need to be addressed in primary care practice. Effective treatment can be limited by the failure to recognize depressive mood and clinical depression in practice. This can be very problematic in multimorbid patients, because symptoms of depression are easily misattributed to somatic illness. Even if diagnosed correctly, overall evidence on optimal management of depression in multimorbid patients is scarce [56] and multimorbidity is only poorly reflected in clinical guidelines [57]. Notable exceptions exist: randomised-controlled trials have shown that improvements in depressive symptoms [58-60] and in social activity [61] are achievable in primary practice settings. A stepped care approach in primary care was also shown to be effective to prevent late life depression [62]. Bogner et al. [58] and Katon et al. [59] showed that in patients with depression and a chronic physical condition, outcomes of both can be improved by integrated and collaborative care, where physicians receive guideline-based recommendations for treatment. Katon et al. also showed improvements in quality of life. In the study by Gensichen et al. [60] outcomes of depression were improved by structured telephone interviews to monitor depression symptoms and support for adherence to medication; quality of life, however, did not significantly improve. Although none of these studies addressed social support, Sommers et al. [61] showed increased social activity when involving social workers in collaborative care. These trials suggest that a promising way to improve outcomes in multimorbid patients with depression are integrated care strategies specifically addressing physical and mental conditions as well as social concerns.
Conclusion
Social support influences health-related quality of life, but this association is strongly mediated by depressive mood. This finding can be explained by cognitive appraisal theory in the sense that social support either protects against or predisposes a person to depressive mood when faced with the threat of multiple illnesses, and this, in turn, affects health-related quality of life. Further research needs to integrate multiple psychosocial factors in order to explain health-related quality of life in multimorbid patients. Integrated care models that specifically address somatic, mental and social dimensions are promising in improving outcomes in multimorbid patients and interventional studies assessing all three dimensions are needed. In family practice, GPs should take into account social support (e.g. by family, friends and support groups) as a potential resource and depressive mood as impediment when caring for multimorbid patients.
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
HvdB, KW, HH und IS designed the study. HvdB and MS are principal investigators of the study. KM, JG, CL, HB, WM, SGR-H, SW, BW, H-HK, GS, HH, HvdB, MS and AD participated in study design and implementation. FSW, CG and AD developed the hypothesis and planned statistical analysis. FSW conducted statistical analysis, prepared the first draft of the manuscript and was in charge of manuscript development and finalization. AD and CG were involved in preparation of the manuscript. All authors read and approved the final manuscript.
Pre-publication history
The pre-publication history for this paper can be accessed here:
Contributor Information
Felix S Wicke, Email: felix@stud.uni-frankfurt.de.
Corina Güthlin, Email: guethlin@allgemeinmedizin.uni-frankfurt.de.
Karola Mergenthal, Email: mergenthal@allgemeinmedizin.uni-frankfurt.de.
Jochen Gensichen, Email: Jochen.Gensichen@med.uni-jena.de.
Christin Löffler, Email: christin.loeffler@med.uni-rostock.de.
Horst Bickel, Email: h.bickel@lrz.tu-muenchen.de.
Wolfgang Maier, Email: w.maier@uni-bonn.de.
Steffi G Riedel-Heller, Email: ries@medizin.uni-leipzig.de.
Siegfried Weyerer, Email: Siegfried.Weyerer@zi-mannheim.de.
Birgitt Wiese, Email: Wiese.Birgitt@MH-Hannover.de.
Hans-Helmut König, Email: h.koenig@uke.uni-hamburg.de.
Gerhard Schön, Email: G.Schoen@uke.uni-hamburg.de.
Heike Hansen, Email: h.hansen@uke.uni-hamburg.de.
Hendrik van den Bussche, Email: bussche@uke.uni-hamburg.de.
Martin Scherer, Email: m.scherer@uke.uni-hamburg.de.
Anne Dahlhaus, Email: dahlhaus@allgemeinmedizin.uni-frankfurt.de.
Funding and acknowledgments
The study was funded by the German Federal Ministry of Education and Research (grant numbers 01ET0725-31 and 01ET1006A-K). The authors would like to thank Melanie Schnee and Moritz Geisberger for their helpful comments on the draft as well as Michael Paulitsch for help with statistical questions.
This article is on behalf of the MultiCare Cohort Study Group, which consists of Attila Altiner, Horst Bickel, Wolfgang Blank, Christian Brettschneider, Monika Bullinger, Hendrik van den Bussche, Anne Dahlhaus, Lena Ehreke, Michael Freitag, Angela Fuchs, Jochen Gensichen, Ferdinand Gerlach, Heike Hansen, Sven Heinrich, Susanne Höfels, Olaf von dem Knesebeck, Hans-Helmut König, Norbert Krause, Hanna Leicht, Melanie Luppa, Wolfgang Maier, Manfred Mayer, Christine Mellert, Anna Nützel, Thomas Paschke, Juliana Petersen, Jana Prokein, Steffi Riedel-Heller, Heinz-Peter Romberg, Ingmar Schäfer, Martin Scherer, Gerhard Schön, Susanne Steinmann, Sven Schulz, Karl Wegscheider, Klaus Weckbecker, Jochen Werle, Siegfried Weyerer, Birgitt Wiese, and Margrit Zieger.
We are grateful to the general practitioners in Bonn, Dusseldorf, Frankfurt/Main, Hamburg, Jena, Leipzig, Mannheim and Munich who supplied the clinical information on their patients.
We also thank Corinna Contenius, Cornelia Eichhorn, Sarah Floehr, Vera Kleppel, Heidi Kubieziel, Rebekka Maier, Natascha Malukow, Karola Mergenthal, Christine Müller, Sandra Müller, Michaela Schwarzbach, Wibke Selbig, Astrid Steen, Miriam Steigerwald, and Meike Thiele for data collection as well as Ulrike Barth, Elena Hoffmann, Friederike Isensee, Leyla Kalaz, Heidi Kubieziel, Helga Mayer, Karine Mnatsakanyan, Michael Paulitsch, Merima Ramic, Sandra Rauck, Nico Schneider, Jakob Schroeber, Susann Schumann, and Daniel Steigerwald for data entry.
References
- Drageset J, Eide GE, Nygaard HA, Bondevik M, Nortvedt MW, Natvig GK. The impact of social support and sense of coherence on health-related quality of life among nursing home residents–a questionnaire survey in Bergen, Norway. Int J Nurs Stud. 2009;46:65–75. doi: 10.1016/j.ijnurstu.2008.07.005. [DOI] [PubMed] [Google Scholar]
- Haring R, Feng Y, Moock J, Volzke H, Dorr M, Nauck M, Wallaschofski H, Kohlmann T. Self-perceived quality of life predicts mortality risk better than a multi-biomarker panel, but the combination of both does best. BMC Med Res Methodol. 2011;11:103. doi: 10.1186/1471-2288-11-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel GL. The need for a new medical model: a challenge for biomedicine. Science. 1977;196:129–136. doi: 10.1126/science.847460. [DOI] [PubMed] [Google Scholar]
- van den Akker M, Buntinx F, Knottnerus JA. Comorbidity or multimorbidity: what's in a name? A review of literature. Eur J Gen Pract. 1996;2:65–70. doi: 10.3109/13814789609162146. [DOI] [Google Scholar]
- Barnett K, Mercer SW, Norbury M, Watt G, Wyke S, Guthrie B. Epidemiology of multimorbidity and implications for health care, research, and medical education: a cross-sectional study. Lancet. 2012;380:37–43. doi: 10.1016/S0140-6736(12)60240-2. [DOI] [PubMed] [Google Scholar]
- Rizza A, Kaplan V, Senn O, Rosemann T, Bhend H, Tandjung R. Age- and gender-related prevalence of multimorbidity in primary care: the Swiss FIRE project. BMC Fam Pract. 2012;13:113. doi: 10.1186/1471-2296-13-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García-Olmos L, Salvador CH, Alberquilla Á, Lora D, Carmona M, García-Sagredo P, Pascual M, Muñoz A, Monteagudo JL, García-López F. Comorbidity patterns in patients with chronic diseases in general practice. PLoS ONE. 2012;7:e32141. doi: 10.1371/journal.pone.0032141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirchberger I, Meisinger C, Heier M, Zimmermann A, Thorand B, Autenrieth CS, Peters A, Ladwig K, Döring A. Patterns of multimorbidity in the aged population. Results from the KORA-Age Study. PLoS ONE. 2012;7:e30556. doi: 10.1371/journal.pone.0030556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schäfer I, von Leitner EC, Schön G, Koller D, Hansen H, Kolonko T, Kaduszkiewicz H, Wegscheider K, Glaeske G, van den Bussche H. Multimorbidity patterns in the elderly: a new approach of disease clustering identifies complex interrelations between chronic conditions. PLoS ONE. 2010;5:e15941. doi: 10.1371/journal.pone.0015941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stolk R, Rosmalen JM, Postma D, Boer R, Navis G, Slaets JJ, Ormel J, Wolffenbuttel BR. Universal risk factors for multifactorial diseases. Eur J Epidemiol. 2008;23:67–74. doi: 10.1007/s10654-007-9204-4. [DOI] [PubMed] [Google Scholar]
- Fortin M, Bravo G, Hudon C, Lapointe L, Almirall J, Dubois M, Vanasse A. Relationship between multimorbidity and health-related quality of life of patients in primary care. Qual Life Res. 2006;15:83–91. doi: 10.1007/s11136-005-8661-z. [DOI] [PubMed] [Google Scholar]
- Fortin M, Lapointe L, Hudon C, Vanasse A, Ntetu A, Maltais D. Multimorbidity and quality of life in primary care: a systematic review. Health Qual Life Outcomes. 2004;2:51. doi: 10.1186/1477-7525-2-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanton AL, Revenson TA, Tennen H. Health psychology: psychological adjustment to chronic disease. Annu Rev Psychol. 2007;58:565–592. doi: 10.1146/annurev.psych.58.110405.085615. [DOI] [PubMed] [Google Scholar]
- Loffler C, Kaduszkiewicz H, Stolzenbach C, Streich W, Fuchs A, van den Bussche H, Stolper F, Altiner A. Coping with multimorbidity in old age - a qualitative study. BMC Fam Pract. 2012;13:45. doi: 10.1186/1471-2296-13-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor SE. In: The Oxford handbook of health psychology. Friedman HS, editor. New York: Oxford University Press; 2011. Social Support: A Review; pp. 189–214. [Google Scholar]
- Sherbourne CD, Meredith LS, Rogers W, Ware JE. Social support and stressful life events: age differences in their effects on health-related quality of life among the chronically ill. Qual Life Res. 1992;1:235–246. doi: 10.1007/BF00435632. [DOI] [PubMed] [Google Scholar]
- Zhou ES, Penedo FJ, Lewis JE, Rasheed M, Traeger L, Lechner S, Soloway M, Kava BR, Antoni MH. Perceived stress mediates the effects of social support on health-related quality of life among men treated for localized prostate cancer. J Psychosom Res. 2010;69:587–590. doi: 10.1016/j.jpsychores.2010.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia H, Uphold CR, Wu S, Reid K, Findley K, Duncan PW. Health-related quality of life among men with HIV infection: effects of social support, coping, and depression. AIDS Patient Care ST. 2004;18:594–603. doi: 10.1089/apc.2004.18.594. [DOI] [PubMed] [Google Scholar]
- Lin N, Ye X, Ensel WM. Social support and depressed mood: a structural analysis. J Health Soc Behav. 1999;40:344–359. doi: 10.2307/2676330. [DOI] [PubMed] [Google Scholar]
- Blazer DG2, Hybels CF. Origins of depression in later life. Psychol Med. 2005;35:1241–1252. doi: 10.1017/S0033291705004411. [DOI] [PubMed] [Google Scholar]
- Theeke LA, Goins RT, Moore J, Campbell H. Loneliness, depression, social support, and quality of life in older chronically ill Appalachians. J Psychol. 2012;146:155–171. doi: 10.1080/00223980.2011.609571. [DOI] [PubMed] [Google Scholar]
- Fortin M, Bravo G, Hudon C, Lapointe L, Dubois M, Almirall J. Psychological distress and multimorbidity in primary care. Ann Fam Med. 2006;4:417–422. doi: 10.1370/afm.528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moussavi S, Chatterji S, Verdes E, Tandon A, Patel V, Ustun B. Depression, chronic diseases, and decrements in health: results from the World Health Surveys. Lancet. 2007;370:851–858. doi: 10.1016/S0140-6736(07)61415-9. [DOI] [PubMed] [Google Scholar]
- Wiesmann U, Niehörster G, Hannich H. Subjective health in old age from a salutogenic perspective. Br J Health Psychol. 2009;14:767–787. doi: 10.1348/135910709X413124. [DOI] [PubMed] [Google Scholar]
- Noël PH, Williams JW, Unützer J, Worchel J, Lee S, Cornell J, Katon W, Harpole LH, Hunkeler E. Depression and comorbid illness in elderly primary care patients: impact on multiple domains of health status and well-being. Ann Fam Med. 2004;2:555–562. doi: 10.1370/afm.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spangenberg L, Forkmann T, Brahler E, Glaesmer H. The association of depression and multimorbidity in the elderly: implications for the assessment of depression. Psychogeriatrics. 2011;11:227–234. doi: 10.1111/j.1479-8301.2011.00375.x. [DOI] [PubMed] [Google Scholar]
- Licht-Strunk E, van Marwijk HWJ, Hoekstra T, Twisk JWR, de Haan M, Beekman ATF. Outcome of depression in later life in primary care: longitudinal cohort study with three years' follow-up. BMJ. 2009;338:a3079. doi: 10.1136/bmj.a3079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunn JM, Ayton DR, Densley K, Pallant JF, Chondros P, Herrman HE, Dowrick CF. The association between chronic illness, multimorbidity and depressive symptoms in an Australian primary care cohort. Soc Psychiatry Psychiatr Epidemiol. 2012;47:175–184. doi: 10.1007/s00127-010-0330-z. [DOI] [PubMed] [Google Scholar]
- Bekele T, Rourke SB, Tucker R, Greene S, Sobota M, Koornstra J, Monette L, Rueda S, Bacon J, Watson J, Hwang SW, Dunn J, Guenter D. The Positive Spaces Healthy Places Team. Direct and indirect effects of perceived social support on health-related quality of life in persons living with HIV/AIDS. AIDS Care. 2012;25:1–10. doi: 10.1080/09540121.2012.701716. [DOI] [PubMed] [Google Scholar]
- Schäfer I, Hansen H, Schon G, Maier W, Hofels S, Altiner A, Fuchs A, Gerlach F, Petersen J, Gensichen J, Schulz S, Riedel-Heller S, Luppa M, Weyerer S, Werle J, Bickel H, Barth K, Konig H, Rudolph A, Wiese B, Prokein J, Bullinger M, von dem Knesebeck O, Eisele M, Kaduszkiewicz H, Wegscheider K, van den Bussche H. The German MultiCare-study: Patterns of multimorbidity in primary health care - protocol of a prospective cohort study. BMC Health Serv Res. 2009;9:145. doi: 10.1186/1472-6963-9-145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schäfer I, Hansen H, Schon G, Hofels S, Altiner A, Dahlhaus A, Gensichen J, Riedel-Heller S, Weyerer S, Blank W, Konig HH, von dem Knesebeck O, Wegscheider K, Scherer M, van den Bussche H, Wiese B. The influence of age, gender and socio-economic status on multimorbidity patterns in primary care. First results from the MultiCare Cohort study. BMC Health Serv Res. 2012;12:89. doi: 10.1186/1472-6963-12-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yesavage JA, Brink TL, Rose TL, Lum O, Huang V, Adey M, O von L. Development and validation of a geriatric depression screening scale: a preliminary report. J Psychiat Res. 1982;17:37–49. doi: 10.1016/0022-3956(82)90033-4. [DOI] [PubMed] [Google Scholar]
- Creed F, Dickens C. In: Depression and physical illness. Steptoe A, editor. Cambridge: Cambridge University Press; 2007. Depression in the medically il; pp. 3–18. [Google Scholar]
- McDowell I. Measuring health: A guide to rating scales and questionnaires. 3. Oxford: Oxford Univ. Press; 2006. [Google Scholar]
- Mitchell AJ, Bird V, Rizzo M, Meader N. Diagnostic validity and added value of the geriatric depression scale for depression in primary care: a meta-analysis of GDS30 and GDS15. J Affect Disorders. 2010;125:10–17. doi: 10.1016/j.jad.2009.08.019. [DOI] [PubMed] [Google Scholar]
- Gauggel S, Birkner B. Validität und Reliabilität einer deutschen Version der Geriatrischen Depressionsskala (GDS) Z Klin Psychol Psych. 1999;28:18–27. doi: 10.1026//0084-5345.28.1.18. [DOI] [Google Scholar]
- Wiczinski E, Döring A, John J, Lengerke T. Obesity and health-related quality of life: does social support moderate existing associations? Br J Health Psychol. 2009;14:717–734. doi: 10.1348/135910708X401867. [DOI] [PubMed] [Google Scholar]
- Knoll N, Kienle R. Fragebogenverfahren zur Messung verschiedener Komponenten sozialer Unterstützung: ein Überblick. Z Med Psychol. 2007;16:57–71. [Google Scholar]
- Fydrich T, Sommer G, Brähler E. F-SozU - Handbuch. http://www.unifr.ch/ztd/HTS/inftest/WEB-Informationssystem/de/4de001/4ca9c6dbe5964b329f6fc7fb5cd864b7/hb.htm.
- Rabin R, Oemar M, Oppe M. EQ-5D-3L User Guide. Basic information on how to use the EQ-5D-3L instrument. [ http://www.euroqol.org/about-eq-5d/publications/user-guide.html]
- Greiner W, Weijnen T, Nieuwenhuizen M, Oppe S, Badia X, Busschbach J, Buxton M, Dolan P, Kind P, Krabbe P, Ohinmaa A, Parkin D, Roset M, Sintonen H, Tsuchiya A, Charro F. A single European currency for EQ-5D health states. Eur J Health Econ. 2003;4:222–231. doi: 10.1007/s10198-003-0182-5. [DOI] [PubMed] [Google Scholar]
- Szende A, Oppe M, Devlin NJ. EQ-5D value sets: Inventory, comparative review, and user guide. Dordrecht: Springer; 2007. [Google Scholar]
- Brazier JE, Walters SJ, Nicholl JP, Kohler B. Using the SF-36 and Euroqol on an elderly population. Qual Life Res. 1996;5:195–204. doi: 10.1007/BF00434741. [DOI] [PubMed] [Google Scholar]
- Brauns H, Steinmann S. Educational reform in France, West-Germany and the United Kingdom: updating the CASMIN Educational Classification. ZUMA-Nachrichten. 1999;44:7–44. [Google Scholar]
- Huntley A, Johnson R, Purdy S, Valderas J, Salisbury C. Measures of multimorbidity and morbidity burden for use in primary care and community settings: a systematic review and guide. Ann Fam Med. 2012;10:134–141. doi: 10.1370/afm.1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron RM, Kenny DA. The moderator-mediator variable distinction in social psychological research: conceptual, strategic, and statistical considerations. J Pers Soc Psychol. 1986;51:1173–1182. doi: 10.1037//0022-3514.51.6.1173. [DOI] [PubMed] [Google Scholar]
- Wu A, Zumbo B. Understanding and using mediators and moderators. Soc Indic Res. 2008;87:367–392. doi: 10.1007/s11205-007-9143-1. [DOI] [Google Scholar]
- Preacher KJ, Leonardelli GJ. Calculation for the Sobel test: An interactive calculation tool for mediation tests. http://quantpsy.org/sobel/sobel.htm.
- van den Akker M, Vos R, Knottnerus JA. In an exploratory prospective study on multimorbidity general and disease-related susceptibility could be distinguished. J Clin Epidemiol. 2006;59:934–939. doi: 10.1016/j.jclinepi.2006.02.009. [DOI] [PubMed] [Google Scholar]
- Lazarus R, Cohen F. In: Health psychology: a handbook. Theories, applications, and challenges of a psychological approach to the health care system. 1. Stone GC, Adler NE, Cohen F, editor. San Francisco: Jossey-Bass; 1979. Coping with the Stresses of Illness; pp. 217–254. [Google Scholar]
- Vogel I, Miksch A, Goetz K, Ose D, Szecsenyi J, Freund T. The impact of perceived social support and sense of coherence on health-related quality of life in multimorbid primary care patients. Chronic Illn. 2012. [DOI] [PubMed]
- Warner L, Schuz B, Wurm S, Ziegelmann J, Tesch-Romer C. Giving and taking–differential effects of providing, receiving and anticipating emotional support on quality of life in adults with multiple illnesses. J Health Psychol. 2010;15:660–670. doi: 10.1177/1359105310368186. [DOI] [PubMed] [Google Scholar]
- Schwarzer R, Knoll N. Functional roles of social support within the stress and coping process: a theoretical and empirical overview. Int J Psychol. 2007;42:243–252. doi: 10.1080/00207590701396641. [DOI] [Google Scholar]
- Kendler KS, Kuhn J, Prescott CA. The interrelationship of neuroticism, sex, and stressful life events in the prediction of episodes of major depression. Am J Psychiatry. 2004;161:631–636. doi: 10.1176/appi.ajp.161.4.631. [DOI] [PubMed] [Google Scholar]
- Swickert R, Owens T. The interaction between neuroticism and gender influences the perceived availability of social support. Pers Indiv Differ. 2010;48:385–390. doi: 10.1016/j.paid.2009.10.033. [DOI] [Google Scholar]
- Smith SM, Soubhi H, Fortin M, Hudon C, O'Dowd T. Managing patients with multimorbidity: systematic review of interventions in primary care and community settings. BMJ. 2012;345:e5205. doi: 10.1136/bmj.e5205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tinetti ME, Bogardus ST Jr, Agostini JV. Potential pitfalls of disease-specific guidelines for patients with multiple conditions. N Engl J Med. 2004;351:2870–2874. doi: 10.1056/NEJMsb042458. [DOI] [PubMed] [Google Scholar]
- Bogner HR, Morales KH, de Vries HF, Cappola AR. Integrated management of Type 2 Diabetes Mellitus and depression treatment to improve medication adherence: a randomized controlled trial. Ann Fam Med. 2012;10:15–22. doi: 10.1370/afm.1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katon WJ, Lin EH, Von Korff M, Ciechanowski P, Ludman EJ, Young B, Peterson D, Rutter CM, McGregor M, McCulloch D. Collaborative care for patients with depression and chronic illnesses. N Engl J Med. 2010;363:2611–2620. doi: 10.1056/NEJMoa1003955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gensichen J, von Korff M, Peitz M, Muth C, Beyer M, Güthlin C, Torge M, Petersen JJ, Rosemann T, König J, Gerlach FM. Case management for depression by health care assistants in small primary care practices: a cluster randomized trial. Ann Intern Med. 2009;151:369–378. doi: 10.7326/0003-4819-151-6-200909150-00001. [DOI] [PubMed] [Google Scholar]
- Sommers LS, Marton KI, Barbaccia JC, Randolph J. Physician, nurse, and social worker collaboration in primary care for chronically ill seniors. Arch Intern Med. 2000;160:1825–1833. doi: 10.1001/archinte.160.12.1825. [DOI] [PubMed] [Google Scholar]
- Van't Veer-Tazelaar P, Smit F, van Hout H, van Oppen P, van der Horst H, Beekman A, van Marwijk H. Cost-effectiveness of a stepped care intervention to prevent depression and anxiety in late life: randomised trial. Br J Psychiatry. 2010;196:319–325. doi: 10.1192/bjp.bp.109.069617. [DOI] [PubMed] [Google Scholar]


