Abstract
Frailty is a physiologic state of increased vulnerability to stressors that results from decreased physiologic reserves or dysregulation of multiple physiologic systems. The construct of frailty has been operationalized as a composite of poor physical function, exhaustion, low physical activity, and weight loss. Several studies have now examined the prevalence of frailty among chronic kidney disease (CKD) or end-stage renal disease (ESRD) patients and have found frailty to be more common among individuals with CKD than among those without. Furthermore, frailty is associated with adverse outcomes among incident dialysis patients, including higher risk of hospitalization and death. Recent evidence shows that frail patients are started on dialysis earlier (at a higher estimated glomerular filtration rate [eGFR]) on average than nonfrail patients but it is unclear whether these patients’ frailty is a result of uremia or is independent of CKD. The survival disadvantage that has been associated with early initiation of dialysis in observational studies could be mediated in part through confounding on the basis of unmeasured frailty. However, available data do not suggest improvement in frailty upon initiation of dialysis; rather, the trajectory appears to be towards higher levels of dependence in activities of daily living (ADLs) after dialysis initiation. Overall, there is no data to suggest that frail patients derive any benefit from early initiation of dialysis either in the form of improved survival or functional status.
What is frailty and why is it important in CKD and ESRD?
Frailty can be defined as a physiologic state of increased vulnerability to stressors that results from decreased physiologic reserves or dysregulation of multiple physiologic systems (1). The prevailing view is that age- or disease-associated physiologic decrements, each of which individually might not reach clinical significance, affect multiple systems and accumulate to reach an aggregate threshold that can be detected as frailty. Frail individuals are then vulnerable to adverse outcomes that can include disability, dependency, falls, institutionalization, hospitalization, and death.
Several operational frailty constructs have been proposed (2–5). These have in common that multiple criteria must be met in order to classify individuals as frail, but they vary widely in the number and types of criteria included. Most or all of these frailty paradigms include measures of physical performance or self-reported functioning as well as some indicator of fatigue or exhaustion, and some also include measures of comorbidity and disability. Originally developed and validated in the Cardiovascular Health Study (CHS) by Fried and colleagues (4,6), perhaps the most widely used single definition is based on at least three of the following five criteria: greater than 10 pounds of unintentional weight loss over one year, weak grip strength, self-report of exhaustion, slow gait speed, and low physical activity. However, because this definition relies on direct measures of physical performance, several modified definitions have been proposed and validated, usually substituting a patient report of functioning for the walk time and grip strength criteria and sometimes eliminating the weight loss criterion or substituting low body mass index (6).
Frailty might be expected to be more common among patients with chronic kidney disease (CKD) or end-stage renal disease (ESRD) than among individuals without CKD because of disease-related and disease-associated conditions such as protein energy wasting, anemia, inflammation, acidosis, and hormonal disturbances, among others (7). Several studies have now examined the prevalence of frailty among chronic kidney disease (CKD) or end-stage renal disease (ESRD) patients using these definitions (8–11) and have found frailty to be more common among individuals with CKD than among those without CKD. Shlipak et al. and Willhelm-Leen et al. used population-based cohorts to examine mainly early stages of CKD (9,10). CHS participants with chronic renal insufficiency (CRI), defined as a serum creatinine above 1.5 mg/dL for men and 1.3 mg/dL for women, had a prevalence of frailty of 15% compared to 6% among individuals without CRI. Among National Health and Nutrition Examination Survey (NHANES) participants aged 20 to 81 years, all stages of CKD, including microalbuminuria with preserved kidney function (estimated glomerular filtration rate [eGFR] > 60 ml/min/1.73m2), were associated with significantly higher odds of frailty compared to individuals without CKD, and the OR was highest among the small percentage of individuals with more advanced CKD (eGFR < 45 ml/min/1.73m2; OR 5.88, 95% CI 3.4 to 10.2). In a cohort of 336 patients with stage 1–4 CKD followed in a CKD clinic with a mean eGFR of 51 ml/min/1.73m2, Roshanravan et al. found a 14% prevalence of frailty (8), which was twice that of the older CHS cohort (1,12,13). Finally, using a national cohort of incident dialysis patients, Johansen et al. reported that 2/3 of the patients met criteria for frailty, including over 40% of the patients who were under 40 years of age and over ¾ of patients over age 60 (11). Furthermore, frail patients had more than a two-fold higher risk of death (HR 2.24, 95% CI 1.60 to 3.15) than those who were not frail even after adjustment for other predictors of death.
We are not aware of longitudinal studies reporting on the development of frailty as CKD progresses, but these data suggest that the prevalence of frailty increases as GFR declines and that frailty is not rare even among younger patients with ESRD. In addition, despite the extremely high prevalence of frailty among patients initiating dialysis, it is still an important construct because it is highly associated with adverse outcomes.
Is frailty associated with timing of dialysis initiation?
Initiation of dialysis in the United States has been occurring at progressively higher levels of eGFR over the last 15 years (14). This trend may be driven by clinical practice guidelines encouraging this practice (12,13). Hemodialysis adequacy guidelines published in 1997 recommended initiating dialysis when eGFR fell below 10.5 ml/min/1.73m2 unless patients’ nutritional status and protein intake were well preserved and patients were free of uremic signs or symptoms, and this recommended eGFR for dialysis initiation was higher than typical dialysis start points prior to that time (12). A subsequent update stated that “when patients reach stage 5 CKD (estimated GFR < 15 mL/min/1.73 m2), nephrologists should evaluate the benefits, risks, and disadvantages of beginning kidney replacement therapy.” These guidelines effectively set an even higher threshold for considering dialysis initiation and further suggested that “particular clinical considerations and certain characteristic complications of kidney failure may prompt initiation of therapy before stage 5″ (13).
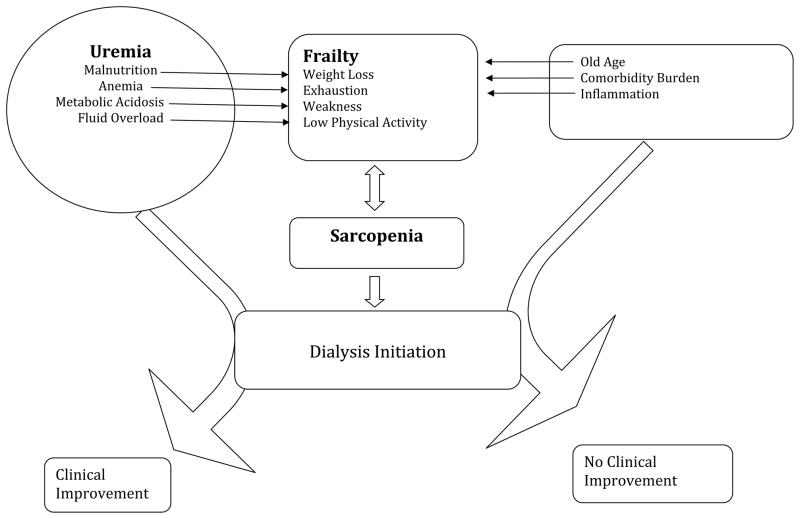
There are several reasons to expect that frail patients might be initiated on dialysis earlier (at higher eGFR) than non-frail patients. First, frailty criteria such as weight loss, fatigue, and poor physical function, can overlap with signs and symptoms of uremia. In this regard, two scenarios are possible (Figure). Uremia could lead to signs and symptoms that meet the criteria for frailty, including malnutrition and weight loss, fatigue, and/or poor physical function, perhaps in part due to volume overload, anemia or other CKD-related complications. These signs and symptoms would then lead to initiation of dialysis, perhaps at a higher eGFR than for patients without these symptoms. Indeed, clinical practice guidelines on Nutrition in CKD advocate that dialysis should be initiated if low nutrient intake leads to development or persistence of protein-energy malnutrition despite attempts to optimize protein-energy intake (15). Kurella Tamura et al. found evidence that this occurs in clinical practice (16). They studied correlates of early dialysis initiation among nursing home residents in the U.S. and found that weight loss, loss of independence in activities of daily living (ADL), and volume overload, all of which might lead to fulfillment of frailty criteria, were associated with earlier dialysis start after adjusting for patient demographics and comorbidity (16).
Figure 1.
Figure Model of Frailty and Uremia Leading to Dialysis Initiation.
Alternatively, patients with advanced CKD may develop frailty independent of their CKD. The components of the frailty phenotype could then be interpreted as uremic symptoms, leading to earlier dialysis start (Figure). Data from the United States Renal Data System (USRDS) support this possibility, showing that the largest increases in eGFR at dialysis initiation have occurred in patients aged 75 and older and among patients with more comorbid conditions, groups in whom the prevalence of frailty would be expected to be particularly high (17). A recent study of patients returning to dialysis after a failed kidney transplant also found that patients with diabetes and peripheral vascular disease were more likely to restart dialysis at higher eGFR than patients without these comorbidities (18).
The potential importance of the distinction between uremia leading to frailty vs. frailty mimicking uremic symptoms lies in the expected outcome of dialysis initiation. If the symptoms are truly related to uremia, then they might be expected to improve with treatment of uremia through initiation of dialysis, but if they are from other causes, symptomatic improvement would be less likely, but any risks or negative consequences of dialysis initiation would still ensue.
Another possible reason for an association between frailty and early dialysis initiation is overestimation of eGFR among frail individuals, leading to what might be considered “pseudo-early” initiation of dialysis. Implicit in the frailty construct is that loss of muscle mass is a central underlying event (4). Thus, frail individuals may have lower levels of creatinine generation (and thus lower serum creatinine concentration) because of lower muscle mass. The resultant overestimation of eGFR by creatinine-based equations could lead to an apparent association between frailty and higher eGFR at dialysis initiation.
A study by Bao et al. directly examined associations between frailty and timing of dialysis initiation using data from the Comprehensive Dialysis Study, which enrolled patients initiating dialysis in a random sample of U.S. dialysis facilities between 2005 and 2007 (19). Frailty was determined based on patients’ report of exhaustion, poor physical function and low physical activity, and GFR was estimated using the 4-variable Modification of Diet in Renal Disease (MDRD) equation (20). The mean age of participants was 60 years, and the overall prevalence of frailty was high at 73%. Patients starting dialysis at higher eGFRs were more likely to be frail (OR 1.08, 95% CI 1.04 to 1.11 for every 1 ml/min/1.73m2 higher eGFR). It is worth noting that the definition of frailty used in this study did not include weight loss because these data were not available. Therefore, weight loss per se was not the cause of the association between frailty and higher eGFR at dialysis initiation. The extent to which overestimation of eGFR was a factor was not addressed in the Bao study, but each individual component of frailty was associated with higher eGFR, including patients’ report of exhaustion, which should not be a function of muscle mass (unpublished data).
What happens to frailty when dialysis is initiated?
There is a paucity of information on the effects of dialysis initiation on frailty, but a few studies have examined functional outcomes after the start of dialysis among elderly individuals (21,22). Kurella Tamura et al. examined the effect of dialysis initiation on functional status in a national sample of nursing home residents starting treatment with dialysis between June 1998 and October 2000 (21). Functional status was measured by assessing the degree of dependence in activities of daily living (ADL). By 12 months after the initiation of dialysis, 58% of the patients had died, and only 13% had maintained predialysis levels of functional status. Furthermore, in a random-effects model, initiation of dialysis was associated with a sharp decline in functional status.
In a single-center cohort study of 97 patients 80 years of age or older in whom long-term dialysis was initiated between 2000–2005, Jassal et al. examined loss of independent function over one year (22). At the time of dialysis initiation, 78% of the cohort was independent (living at home with no assistance with ADLs), but after one year this percentage had decreased to 23%, with the remainder having been admitted to an assisted-living setting or nursing home, submitted an application for caregiver support, or died.
Although neither of these studies addressed frailty per se, they provide strong evidence to suggest that frailty does not improve when dialysis is initiated. Rather, the further decline in functional status after dialysis initiation in both studies indicates that dialysis may be associated with progression of frailty towards loss of independence. The populations included in both cohorts would be expected to have a high prevalence of frailty, since frailty is often a precursor to loss of independence, an outcome the patients in the Kurella Tamura study had already experienced. Similarly, given the strong association of age with frailty and the high prevalence of frailty reported in unselected incident dialysis patients (11,19), the very elderly patients in the Jassal study were likely to have an extremely high prevalence of frailty.
Does frailty mediate the association between early dialysis initiation and bad outcomes?
Numerous observational studies have examined the association of early start of dialysis and survival in many different countries and settings over the last decade with remarkable consensus that higher eGFR at dialysis initiation is associated with worse survival (23–32). Nevertheless, the observational nature of the data have led to concern over confounding by indication, wherein sicker patients are initiated on dialysis earlier and have higher mortality. Statistical approaches to account for this confounding, such as adjustment for comorbidity, use of propensity scores, and restriction to a “healthier” subset of patients have failed to eliminate the apparent survival disadvantage of early dialysis start. However, the only randomized trial of early vs. late dialysis initiation strategies did not find a disadvantage (nor an advantage) to early start (33), further highlighting the possibility of residual confounding.
Could frailty be an important confounder not considered in prior studies? Many of the studies showing worse outcomes with early initiation of dialysis have been based on registry data, which does not contain extensive measures of physical function or other data necessary to determine whether patients are frail. However, the Comprehensive Dialysis Study (CDS) included a patient questionnaire that assessed self-reported physical function, fatigue/exhaustion, and physical activity (34). Frailty was associated with higher eGFR at dialysis initiation in this cohort and also with higher mortality during 2.9 years of follow-up (HR 1.79, 95% CI 1.44 to 2.24) (19). Higher eGFR was associated with higher mortality (HR 1.24, 1.14 to 1.36 per 5 ml/min/1.73m2) when frailty was not included in the multivariable model, but addition of frailty attenuated the association between eGFR and mortality such that it was no longer statistically significant (HR 1.08, 95% CI 0.98 to 1.19). These results are consistent with the possibility that frailty serves as a marker of the tendency to start dialysis earlier among sicker patients and that this tendency is responsible for some or all of the observed harm associated with higher eGFR at the start of dialysis. These findings could also be consistent with overestimation of eGFR in these frail patients so that they appeared to have a higher eGFR at dialysis start, and since body composition was not measured in CDS, this possibility cannot be eliminated.
It is important to note that although higher eGFR at dialysis initiation was not associated with significantly higher mortality after adjustment for frailty, this adjustment did not uncover a survival advantage to early dialysis initiation. Rather, the lack of a clear association of eGFR with survival was in agreement with the only randomized controlled trial to examine the question of timing of dialysis initiation to date (33,35).
Future areas for research
There is a need for more research into all of the topics covered in this review. First, studies of frailty in CKD and ESRD have used many different specific definitions of frailty, and studies should attempt to determine which definition or definitions are most useful, balancing practicality and predictive power. Measuring physical performance requires more time and effort, but it is currently uncertain whether definitions of frailty based on physical performance better identify individuals at high risk for adverse outcomes than those based on more accessible questionnaire data. Second, with a useful definition of frailty in hand, studies should assess the course of frailty with progression of CKD and dialysis initiation. Estimation of GFR using cystatin C could be useful to alleviate the potential for misclassification of CKD stage among frail individuals with low muscle mass. Finally, outcomes after dialysis initiation should be studied. Frailty should be examined both as a predictor of adverse outcomes such as hospitalization and mortality and as an outcome in its own right. Can frailty be reversed among patients with ESRD? Does frailty progress after initiation of dialysis? Does the choice of dialysis modality have an impact on the trajectory of frailty? Does the timing of dialysis initiation have any impact on frailty or on other outcomes among frail individuals who begin dialysis?
Conclusions
In conclusion, the limited available data suggest that frailty is associated with earlier start of dialysis, but the actual causal pathways among uremia, frailty, and earlier dialysis initiation are yet to be determined. Based on currently available observational data (21,22) and limited data from a randomized clinical trial (33,36), frailty does not improve with dialysis initiation. Examining frailty among patients starting dialysis may shed some light on the associations of early initiation of dialysis and higher mortality, and frailty may account, in part, for the observed association between higher eGFR and higher mortality. Nevertheless, there is no indication that early initiation of dialysis is beneficial among frail (or nonfrail) patients.
Table 1.
Table Definitions of frailty used or adapted for use in studies of patients with CKD or ESRD
| Study | Frailty Definition | Sample | N | Age | Details of results |
|---|---|---|---|---|---|
| Fried4 (2001) |
Slowness/Weakness, 2 points: Timed 15-foot walk (slowest 20%); grip strength (lowest 20%) Poor Endurance/Exhaustion, 1 point: Two items from the Center for Epidemiologic Studies Depression (CES-D) Scale: Everything I do is an effort. I cannot get going. Answers >3–4 days/wk to either question were scored positive for frailty Physical Activity, 1 point: Modified Minnesota Leisure Time Activities (MMLTA): Self report of whether a person performed any if 18 activities in the prior week. Kcal of energy expended in a week on leisure time activity was calculated. Those in the bottom quintile were deemed positive for frailty. (men exerting <383kcal;women exerting <270 kcal/week were scored positive for frailty.) Unintentional Weight Loss, 1 point: Self-reported weight loss>10 pounds in previous year at baseline. |
Cardiovascular Health Study (community-dwelling elderly) | 5888 | >65 (mean 76) |
|
| Woods6 (2005) |
Slowness/Weakness, 2 points: Rand-36 Physical Function scale (score<75) Poor Endurance/Exhaustion, 1 point: Rand-36 Vitality Scale (score<55). Over past 4 weeks: Did you feel worn out? Did you feel tired? Did you have a lot of energy? Did you feel full of pep? Physical Activity, 1 point: Detailed activity questionnaire assessing frequency and duration of walking and mild, moderate, and strenuous activities. Kcal of weekly energy expenditure was calculated, and those in the lowest quartile were scored positive for frailty. Unintentional Weight Loss not included. |
Women’s Health Initiative (women, aged 65 to 79) | 65–79 |
|
|
| Johansen11 (2007) |
Slowness/Weakness, 1 point: Rand-36 Physical Function scale (score<75) Poor Endurance/Exhaustion, 1 point: Rand-36 Vitality Scale (score<55) Physical Activity, 1 point: How often do you exercise (do physical activity during your leisure time)? Daily or almost daily 4 to 5 times a week 2 to 3 times a week. Almost never or never individuals answering “almost never or never” were classified as inactive. Undernourished or cachectic (1 point): As assessed by the individual completing the CMS Form 2728. |
Dialysis Morbidity and Mortality Study, Wave II (incident dialysis patients) | 2275 | >18 (mean 58) |
|
| Bao19 (2011) |
Slowness/Weakness, 1 points: Rand-12 Physical Function scale (score<75) Poor Endurance/Exhaustion, 1 point: Rand-12 Vitality Scale (score<55) Physical Activity, 1 point: Lowest quintile of Adjusted Activity Score of the Human Activity Profile from normative data stratified by age and sex. |
Comprehensive Dialysis Study, incident dialysis patients | 1576 | >18 (mean 60) |
|
| Roshanravan8 (2012) |
Slowness/Weakness, 2 points: Timed 4-m walk (slowest 20% [i.e., same absolute cutoffs] from the CHS); grip strength (lowest 20% from the CHS) Poor Endurance/Exhaustion, 1 point: Lowest 20th percentile exhaustion score on SF-36 derived from adults >65 years of age (<37.5 on the Vitality scale) Physical Activity, 1 point: Self-reported exercise < 1x/wk Unintentional Weight Loss, 1 point: Self-reported weight loss>10 pounds in previous 6 mos. |
Seattle Kidney Study (patients with CKD not on dialysis) | 336 | >18 (mean 59) |
|
Abbreviations: CKD, chronic kidney disease; ESRD, end-stage renal disease; ADL, activities of daily living
Acknowledgments
Sources of support: Dr. Johansen is supported by 1K24DK085153 from the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK). Dr. Delgado is supported by a VA Career Development Award.
Footnotes
conflict of interest: The authors declare no potential financial conflicts of interested related to the material presented in this manuscript.
References
- 1.Fried LP, Ferrucci L, Darer J, Williamson JD, Anderson G. Untangling the concepts of disability, frailty, and comorbidity: implications for improved targeting and care. J Gerontol Med Sci. 2004;59:255–263. doi: 10.1093/gerona/59.3.m255. [DOI] [PubMed] [Google Scholar]
- 2.Ensrud KE, Ewing SK, Taylor BC, Fink HA, Cawthon PM, Stone KL, Hillier TA, Cauley JA, Hochberg MC, Rodondi N, Tracy JK, Cummings SR. Group for the Study of Osteoporotic Fractures Research: Comparison of 2 frailty indexes for prediction of falls, disability, fractures, and death in older women. Arch Intern Med. 2008;168:382–389. doi: 10.1001/archinternmed.2007.113. [DOI] [PubMed] [Google Scholar]
- 3.Rolfson DB, Majumdar SR, Tsuyuki RT, Tahir A, Rockwood K. Validity and reliability of the Edmonton frail scale. Age Ageing. 2006;35:526–529. doi: 10.1093/ageing/afl041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, Seeman T, Tracy R, Kop WJ, Burke G, McBurnie MA. Group for the Cardiovascular Health Study Collaborative Research: Frailty in older adults: evidence for a phenotype. J Gerontol Med Sci. 2001;56A:M146–M156. doi: 10.1093/gerona/56.3.m146. [DOI] [PubMed] [Google Scholar]
- 5.Jones DM, Xiaowei Song, Rockwood K. Operationalizing a frailty index from a standardized comprehensive geriatric assessment. J Am Geriatr Soc. 2004;52:1929–1933. doi: 10.1111/j.1532-5415.2004.52521.x. [DOI] [PubMed] [Google Scholar]
- 6.Woods NF, LaCroix AZ, Gray SL, Aragaki A, Cochrane BB, Brunner RL, Masaki K, Murray A, Newman AB. Frailty: emergence and consequences in women aged 65 adn older in the Women’s Health Initiative Observational Study. J Am Geriatr Soc. 2005;53:1321–1330. doi: 10.1111/j.1532-5415.2005.53405.x. [DOI] [PubMed] [Google Scholar]
- 7.Kim JC, Kalantar-Zadeh K, Kopple JD. Frailty and protein-energy wasting in elderly patients with end stage kidney disease. J Am Soc Nephrol. 2013;24:337–351. doi: 10.1681/ASN.2012010047. [DOI] [PubMed] [Google Scholar]
- 8.Roshanravan B, Khatri M, Robinson-Cohen C, Levin G, Patel KV, de Boer IH, Seliger S, Ruzinski J, Himmelfarb J, Kestenbaum B. A prospective study of frailty in nephrology-referred patients with CKD. Am J Kidney Dis. 2012;60:912–921. doi: 10.1053/j.ajkd.2012.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Willhelm-Leen ER, Hall YN, Kurella Tamura M, Chertow GM. Frailty and chronic kidney disease: the Third National Health and Nutrition Evaluation Survey. Am J Med. 2009;122:664–671. doi: 10.1016/j.amjmed.2009.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shlipak MG, Stehman-Breen C, Fried LF, Song X, Siscovick D, Fried LP, Psaty BM, Newman AB. The presence of frailty in elderly persons with chronic renal insufficiency. Am J Kidney Dis. 2004;43:861–867. doi: 10.1053/j.ajkd.2003.12.049. [DOI] [PubMed] [Google Scholar]
- 11.Johansen KL, Chertow GM, Jin C, Kutner NG. Significance of frailty among dialysis patients. J Am Soc Nephrol. 2007;18:2960–2967. doi: 10.1681/ASN.2007020221. [DOI] [PubMed] [Google Scholar]
- 12.Foundation National Kidney. NKF-KDOQI Clinical Practice Guidelines. Am J Kidney Dis. 1997;30:S67–S136. doi: 10.1016/s0272-6386(97)70028-3. [DOI] [PubMed] [Google Scholar]
- 13.Foundation National Kidney. KDOQI Clinical Practice Guidelines for Hemodialysis Adequacy, Update 2006. Am J Kidney Dis. 2006;48:S2–S90. doi: 10.1053/j.ajkd.2006.03.051. [DOI] [PubMed] [Google Scholar]
- 14.System U.S. Renal Data. . USRDS 2012 Annual Data Report: Atlas of Chronic Kidney Disease and End-Stage Renal Disease in the United States. Bethesda, MD: National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; 2012. [Google Scholar]
- 15.Foundation National Kidney. NKF KDOQI Clinical Practice Guidelines for Nutrition in Chronic Renal Failure. Am J Kidney Dis. 2000;35:S1–S140. doi: 10.1053/ajkd.2000.v35.aajkd03517. [DOI] [PubMed] [Google Scholar]
- 16.Kurella Tamura M, O’Hare AM, McCulloch CE, Johansen KL. Signs and symptoms associated with earlier dialysis initiation in nursing home residents. Am J Kidney Dis. 2010;56:1117–1126. doi: 10.1053/j.ajkd.2010.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.System U.S. Renal Data. USRDS 2004 Annual Data Report: Atlas of End-Stage Renal Disease in the United States. Bethesda, MD: National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; 2004. [Google Scholar]
- 18.Molnar MZ, Streja E, Kovesdy CP, Hoshino J, Hatamizadeh P, Glassock RJ, Ojo AO, Kalantar-Zadeh K. Estimated glomerular filtration rate at reinitiation of dailysis and mortality in failed kidney transplant recipients. Nephrol Dial Transplant. 2012;27:2913–2921. doi: 10.1093/ndt/gfs004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bao Y, Dalrymple L, Chertow GM, Kaysen GA, Johansen KL. Frailty, dialysis initiation, and mortality in end-stage renal disease. Arch Intern Med. 2012;172:1071–1077. doi: 10.1001/archinternmed.2012.3020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: A new prediction equation. Ann Intern Med. 1999;130:461–470. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 21.Kurella Tamura M, Covinsky KE, Chertow GM, Yaffe K, Landefeld CS, McCulloch CE. Functional status of elderly adults before and after initiation of dialysis. N Engl J Med. 2009;361:1539–1547. doi: 10.1056/NEJMoa0904655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jassal SV, Chiu E, Hladunewich M. Loss of independence in patients starting dialysis at 80 years of age or older. N Engl J Med. 2009;361:1612–1613. doi: 10.1056/NEJMc0905289. [DOI] [PubMed] [Google Scholar]
- 23.Korevaar JC, Jansen MA, Dekker FW, Jager KJ, Boeschoten EW, Krediet RT, Bossuyt PM Group Netherlands Cooperative Study on the Adequacy of Dialysis Study. When to initiate dialysis: effect of proposed US guidelines on survival. Lancet. 2001;358:1046–1050. doi: 10.1016/S0140-6736(01)06180-3. [DOI] [PubMed] [Google Scholar]
- 24.Traynor JP, Simpson K, Geddes CC, Deighan CJ, Fox JG. Early initiation of dialysis fails to prolong survival in patients with end-stage renal failure. J Am Soc Nephrol. 2002;13:2125–2132. doi: 10.1097/01.asn.0000025294.40179.e8. [DOI] [PubMed] [Google Scholar]
- 25.Beddhu S, Samore MH, Roberts MS, Stoddard GJ, Ramkumar N, Pappas LM, Cheung AK. Impact of timing of initiation of dialysis on mortality. J Am Soc Nephrol. 2003;14:2305–2312. doi: 10.1097/01.asn.0000080184.67406.11. [DOI] [PubMed] [Google Scholar]
- 26.Sawhney S, Djurdjev O, Simpson K, Macleod A, Levin A. Survival and dialysis initiation: comparing British Columbia and Scotland registries. Nephrol Dial Transplant. 2009;24:3186–3192. doi: 10.1093/ndt/gfp189. [DOI] [PubMed] [Google Scholar]
- 27.Stel VS, Dekker FW, Ansell D, Augustijn H, Casino FG, Collart F, Finne P, Ioannidis GA, Salomone M, Traynor JP, Zurriaga O, Verrina E, Jager KJ. Residual renal function at the start of dialysis and clinical outcomes. Nephrol Dial Transplant. 2009;24:3175–3182. doi: 10.1093/ndt/gfp264. [DOI] [PubMed] [Google Scholar]
- 28.Lassalle M, Labeeuw M, Frimat L, Villar E, Joyeux V, Couchoud C, Stengel B. Age and comorbidity may explain the paradoxical association of an early dialysis start with poor survival. Kidney Int. 2010;77:700–707. doi: 10.1038/ki.2010.14. [DOI] [PubMed] [Google Scholar]
- 29.Hwang SJ, Yang WC, Lin MY, Mau LW, Chen HC. Nephrology Taiwan Society of: Impact of clinical conditions at dialysis initiation on mortality in incident haemodialysis patients: a national cohort study in Taiwan. Nephrol Dial Transplant. 2010;25:2616–2624. doi: 10.1093/ndt/gfq308. [DOI] [PubMed] [Google Scholar]
- 30.Wright S, Klausner D, Baird B, Williams ME, Steinman T, Tang H, Ragasa R, Goldfarb-Rumyantzev AS. Timing of dialysis initiation and survival in ESRD. Clin J Am Soc Nephrol. 2010;5:1828–1835. doi: 10.2215/CJN.06230909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Clark WF, Na Y, Rosansky SJ, Sontrop JM, Macnab JJ, Glassock RJ, Eggers PW, Jackson K, Moist L. Association between estimated glomerular filtration rate at initiation of dialysis and mortality. CMAJ. 2011;183:47–53. doi: 10.1503/cmaj.100349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rosansky SJ, Eggers PW, Jackson K, Glassock RJ, Clark WF. Early start of hemodialysis may be harmful. Arch Intern Med. 2011;171:396–403. doi: 10.1001/archinternmed.2010.415. [DOI] [PubMed] [Google Scholar]
- 33.Cooper BA, Branley P, Bulfone L, Collins JF, Craig JC, Fraenkel MB, Harris A, Johnson DW, Kesselhut J, Li JJ, Luxton G, Pilmore A, Tiller DJ, Harris DC, Pollock CA Study IDEAL. A randomized, controlled trial of early versus late initiation of dialysis. N Engl J Med. 2010;363:609–619. doi: 10.1056/NEJMoa1000552. [DOI] [PubMed] [Google Scholar]
- 34.Kutner NG, Johansen KL, Kaysen GA, Pederson S, Chen SC, Agodoa LY, Eggers PW, Chertow GM. The comprehensive dialysis study (CDS): a USRDS special study. Clin J Am Soc Nephrol. 2009;4:645–650. doi: 10.2215/CJN.05721108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cooper BA, Branley P, Bulfone L, Collins JF, Craig JC, Dempster J, Fraenkel MB, Harris A, Harris DC, Johnson DW, Kesselhut J, Luxton G, Pilmore A, Pollock CA, Tiller DJ Committee IDEAL Study. Steering: The Initiating Dialysis Early and Late (IDEAL) study: study rationale and design. Perit Dial Int. 2004;24:176–181. [PubMed] [Google Scholar]
- 36.Harris A, Cooper BA, Li JJ, Bulfone L, Branley P, Collins JF, Craig JC, Fraenkel MB, Johnson DW, Kesselhut J, Luxton G, Pilmore A, Rosevaar M, Tiller DJ, Pollock CA, Harris DC. Cost-effectiveness of initiating dialysis early: a randomized controlled trial. Am J Kidney Dis. 2011;57:707–715. doi: 10.1053/j.ajkd.2010.12.018. [DOI] [PubMed] [Google Scholar]