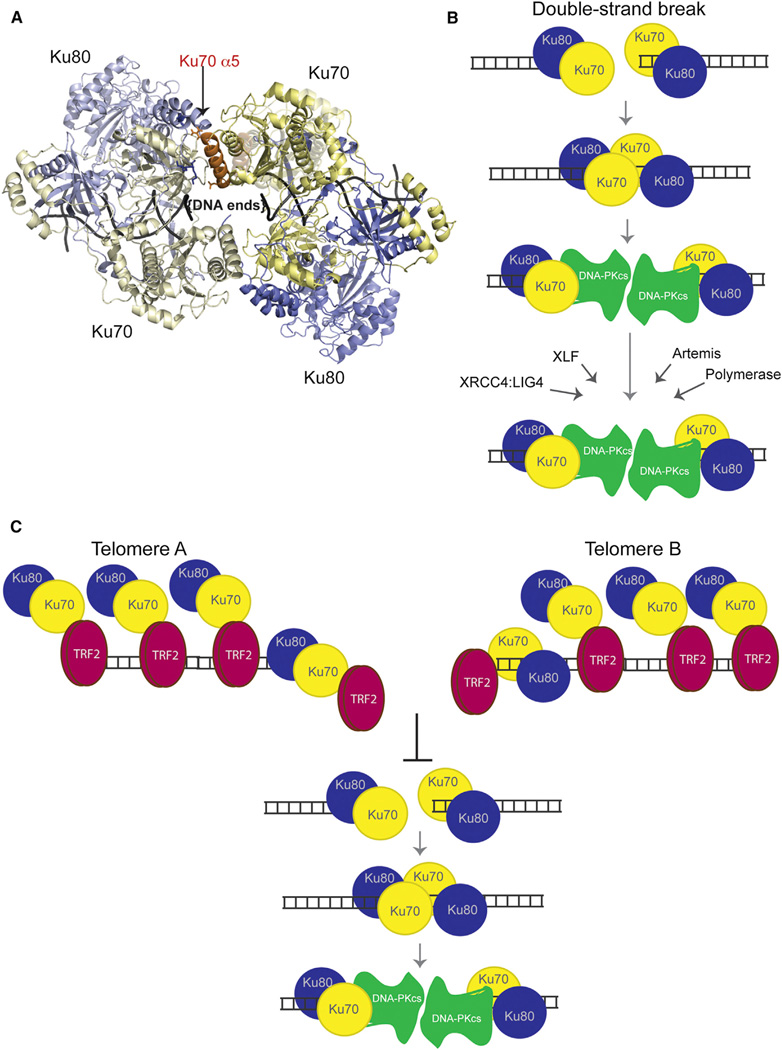
Figure 7. Models for Ku Heterotetramerization and Its Inhibition at Telomeres by TRF2.
(A) Top view of a hypothetical Ku tetramer obtained via protein docking using HADDOCK web server. Yellow: Ku70. Blue: Ku80. Orange: Ku70 α5. Residues R185, D192, and D195 from Ku70 are shown in stick representation. The vWA domain from Ku70 would have to move to accommodate this model of heterotetramerization. Its original position with respect to its native heterodimer is shown in “ghost” (semitransparent) representation.
(B) Mechanistic model for the role of Ku’s heterotetramerization during NHEJ. We propose that Ku heterotetramerization is required in the initial NHEJ steps to synapse the DNA ends of a DSB prior to the DNA-PKcs recruitment. Formation of the DNA-PK complex displaces Ku away from the end, which effectively disassociates the Ku-Ku interaction and allows DNA-PKcs to replace Ku in synapsing the two ends. Later roles of Ku in recruiting NHEJ factors to DSBs would not require heterotetramer formation.
(C) Mechanistic model for the inhibition of Ku’s heterotetramerization by TRF2 at telomeres. We propose that TRF2’s interaction with the Ku70 α5 helix effectively inhibits Ku heterotetramerization at telomeres and the synapsis of telomeric ends, thereby blocking telomeric NHEJ.