Abstract
Background:
Individuals with schizophrenia demonstrate a wide range of social cognitive deficits that significantly compromise functioning. Early visual processing is frequently disrupted in schizophrenia, and growing evidence suggests a role of perceptual dysfunctions in socioemotional functioning in the disorder. This study examined visual integration (the ability to effectively integrate individual, local visual features into a holistic representation), a target construct of basic perception identified by the Cognitive Neuroscience Treatment Research to Improve Cognition in Schizophrenia initiative, and its relationship with eye- contact perception and emotional intelligence in schizophrenia.
Methods:
Twenty-nine participants with schizophrenia (SCZ) and 23 healthy controls (HC) completed tasks measuring visual integration (Coherent Motion Task, Contour Integration Task), an eye-contact perception task, and a measure of emotional intelligence.
Results:
SCZ participants showed compromised visual integration as suggested by poorer performance on the Contour Integration Task relative to HC. Visual integration was a significant predictor of eye-contact perception and emotional intelligence among SCZ. The amounts of variances in these 2 social cognitive areas accounted for by visual integration were comparable to and overlapped with those accounted for by the diagnosis of schizophrenia.
Conclusions:
Individuals with schizophrenia showed compromised visual integration, and this may play a significant role in the observed deficits in higher level processing of social information in the disorder.
Key words: psychosis, visual perception, perceptual organization, social cognition, sensory processing
Introduction
Schizophrenia is a severe mental disorder that often runs a chronic course and significantly influences social functioning. Recent research has shown that individuals with schizophrenia (SCZ) show deficits in a wide range of social cognitive processes, including emotion recognition, eye-contact perception, theory of mind, and social reasoning.1–4 Because social cognition is critical to functional outcomes in SCZ,5 understanding the nature of these processes has become a research priority. Given that many social cognitive processes involve information processing in the visual modality and basic visual perceptual functions are frequently disrupted in schizophrenia,6 it is reasonable to ask whether deficits in visual perception are related to the abnormal social cognitive processes noted in SCZ.
Building on the extensive research on early visual processing in SCZ, some researchers have proposed “cascade models” that suggest disrupted visual perception leads to higher order dysfunctions observed in SCZ. For example, some aspects of visual processing (eg, backward masking and contrast detection) have been correlated with social cognitive deficits, clinical symptoms, and functional impairment in SCZ7–9 though these studies were based on cross-sectional data and alternative models with opposite path directions were not tested, leaving the causal direction of these variables debatable. The Cognitive Neuroscience Treatment Research to Improve Cognition in Schizophrenia (CNTRICS) initiative recently identified 2 functional concepts as the target constructs within the perception domain for further investigations in SCZ.6 This study investigated one of these constructs, visual integration, and its relationship with 2 social cognitive processes, eye gaze processing and emotional intelligence, that have documented deficits in SCZ.
Visual Integration in Schizophrenia
Visual processing involves an interaction between bottom- up signaling of sensory data predetermined by the architecture of the reinta-V1 pathway and its complex lateral and top-down modulations based on context and prior experience. Visual integration is one of the essential processes in this highly interactive processing stream; it concerns the ability to link together individual local attributes of a scene to form a larger, global complex structure in order to guide behavior.6 Examples of visual integration include Gestalt grouping phenomena (ie, perceiving objects as organized patterns based on proximity, similarity, closure, good continuation, etc.) and coherent motion perception. Integration is a particularly useful construct of perception for SCZ research because, in addition to evidence of impairment (discussed below), it can be measured in both humans and animals, thus facilitating translational investigations linking neural circuitry and pharmacology.6
There is strong evidence that visual integration is compromised in SCZ. SCZ participants have shown poorer performance than healthy controls (HC) in object recognition from fragmented images,10 perceptual grouping by proximity and color similarity,11 and contour integration.12,13 Additionally, for tasks in which global integration would normally decrease the accuracy of perception of individual elements, SCZ perform better than HC,14,15 further supporting visual integration deficits in the disorder.
Functional Correlates of Visual Integration
Visual integration impairment may contribute to clinical symptoms and functional deficits in SCZ. Several studies have shown a linkage between visual perception and a range of adaptive functioning16,17 and disorganized symptoms.12 Although a recent large-sample study found no correlation between visual integration and community skills in SCZ,18 this sample was relatively high functioning and had mild visual integration problems. Further, specific social cognitive processes may be more affected than broad measures of functional outcome in SCZ.9,19 For example, the well-documented deficits in face identification and emotion recognition observed in schizophrenia may be related to unsuccessful configural processing of facial features. In fact, event-related brain potential studies have shown that emotion recognition deficits in SCZ are associated with reduced N170, an index of holistic structural encoding of facial features,20,21 but see also Wynn et al for intact N170 during affect recognition in SCZ.22 Further, during gaze discrimination, compared with HC, SCZ participants’ behavioral3 and N170 responses23 are less affected by head orientation, indicating a tendency to integrate less the contextual cue of head orientation in gaze processing. Taken together, visual integration likely plays a role in the processing of important social signals.
The Present Study
To understand the role of visual integration in socioemotional information processing in SCZ, gaze perception and emotional intelligence were selected as the social cognitive processes in this study because they represent different levels of processing and each has documented relevance to SCZ. Specifically, effective use of gaze direction to guide behavior is critical to social adaptation.24 The determination of the self-referential nature of gaze represents a process that intersects the perceptual and interpretation levels,3 and SCZ participants have demonstrated abnormalities such as self-referential bias with ambiguous gaze1,3 and more uncertainty when making eye-contact judgment.3 Emotional intelligence captures broader socioemotional functioning ranging from facial emotion perception to higher level processes including understanding, inferring, and effectively regulating emotional states,25 and SCZ participants have shown consistent deficits in emotional intelligence.3,26,27 In addition, a recent neuroimaging study showed that poorer emotional intelligence in SCZ was associated with abnormal functional coupling of the visual cortex with anterior cingulate cortex during a social appraisal task.28 Although early visual processing was not directly assessed in this study, it provides evidence for an important connection between basic visual processing and higher level social functioning in the disorder, calling for further investigations.
This study aims to elucidate the relationship between visual integration and social cognition in SCZ. Visual integration was measured using a Coherent Motion Task and a Contour Integration Task, as recommended by CNTRICS.29 For social cognition, gaze perception and emotional intelligence were selected to represent a range of levels of socioemotional information processing with documented relevance to SCZ.1,3,26,27
We hypothesized that (1) SCZ participants would show poorer visual integration than HC; (2) visual integration would be associated with social cognition (including gaze perception and emotional intelligence) in SCZ; and (3) variances in the social cognitive measures due to visual integration would be comparable to and overlap with those due to diagnosis group.
Methods
Participants
Thirty-one SCZ and 23 HC completed the study. However, 2 SCZ participants’ performance approached chance level and thus were excluded from the analyses. The remaining 29 SCZ and 23 HC were well matched for age, sex, and parental education (table 1).
Table 1.
Participant Characteristics
| Variable | SCZ (n = 29) (Mean ± SD) |
HC (n = 23) (Mean ± SD) |
t/χ2 | P |
|---|---|---|---|---|
| Age (y) | 43.6±12.6 | 43.5±13.1 | 0.03 | .978 |
| Sex (male/female) | 20/9 | 18/5 | 0.56 | .453 |
| Education (y) | 13.6±1.9 | 16.4±2.6 | −4.41 | <.001 |
| Parental education (y) | 14.7±3.7 | 15.0±2.8 | −0.37 | .715 |
| Age of onset (y) | 19.8±7.0 | — | — | — |
| Duration of illness (y) | 23.8±13.1 | — | — | — |
| SAPS | 5.6±3.6 | — | — | — |
| SANS | 4.5±3.1 | — | — | — |
| CPZeq (mg daily) | 428±489 | — | — | — |
| Dichotomous gaze perception | 2.23±0.68 | 2.65±0.48 | −2.47 | .017 |
| MSCEITa | 90.8±18.4 | 106.5±17.4 | −2.80 | .008 |
Note: SAPS, sum of global subscores on the Scale for the Assessment of Positive Symptoms31; SANS, sum of global scores of the flat affect, alogia, apathy, and anhedonia subscales of the Scale for the Assessment of Negative Symptoms32; CPZeq, antipsychotic dose in chlorpromazine equivalent mg daily; MSCEIT, Age- and gender-adjusted total score on the Mayer-Salovey-Caruso Emotional Intelligence Test.25
aDue to missing data, MSCEIT overall score was available for only 22 SCZ and 19 HC.
SCZ participants were recruited through community advertisements or referrals by clinicians or researchers of local mental health and university clinics. They met criteria for schizophrenia (n = 21) or schizoaffective disorder (n = 8) using the Structured Clinical Interview for DSM-IV Axis-I Disorders (SCID-I).30 Exclusion criteria included inability to give informed consent and a history of substance abuse/dependence in the past 12 months. Nine SCZ were treated with conventional antipsychotics and 19 with atypical; 1 was medication-free.
HC participants were recruited from community advertisements and referrals by other researchers, followed by an initial phone screening and a SCID-I interview. Exclusion criteria included lifetime Axis-I disorders, substance abuse in the past 5 years, lifetime substance dependence, a history of closed head injury or medical conditions that affect brain functions, and history of psychosis and bipolar disorder among first-degree relatives.
The study was conducted in accordance with the protocol approved by the Institutional Review Board of the University of Michigan Medical School. Written informed consent was obtained from each participant after full explanation of the study. All participants were 18–60 years old and had at least 20/30 vision according to a Snellen chart.
Task and Procedure
Visual Integration.
Integration was measured using the Coherent Motion Task (Motion) and the Contour Integration Task (Contour). The tasks were presented using MATLAB 2008 (MathWorks Inc.) in an adaptive approach with a 3-up-1-down staircase method, terminated after 12 reversals (see online supplementary material 1 for details). The mean performance of the last 8 reversals was used as the participant’s threshold. For each task, participants completed a practice session (10 trials with feedback) and then completed the full-length task twice. The mean threshold of the 2 blocks was used in the analyses. The distance between the stimuli and the viewer was controlled by a chin rest placed at 16 inches from the computer screen.
Coherent Motion Task.
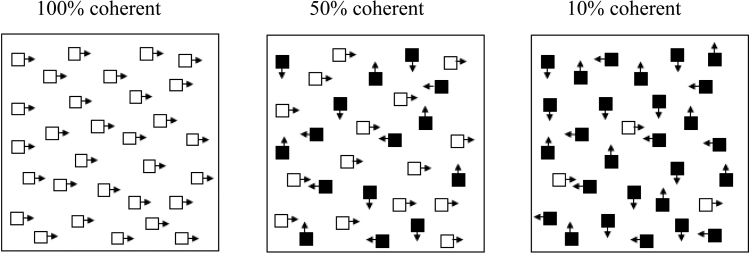
Participants had to identify the direction (up, down, left, or right) of a group of coherently moving dots against a background of randomly moving dots. Difficulty increases as fewer dots move coherently (figure 1). The density of the dots was 0.5 dots per square degree, and the dots moved at 3°/s. In each trial, the stimulus was presented for 1 s, and participants were allowed 5 s to respond. For the first 2 reversals, the percentage of coherent dots changed by 10% per reversal, thereafter, 2.5% per reversal.
Fig. 1.
Coherent Motion Task. Participants had to indicate the direction of the coherent dots (upward, downward, left, or right). This figure shows sample trials with 100%, 50%, and 10% coherence.
Contour Integration Task.
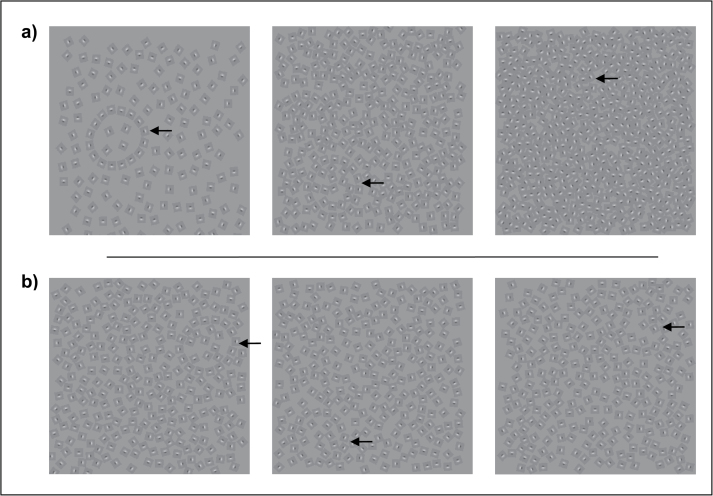
This was a modified version of the original paradigm by Field et al.33 Participants were to locate a contour formed by Gabor elements (left or right on the screen) against a background of noise. Because 2 versions (density version34 and jitter version)35 have been used in schizophrenia research and it is unclear which version is more reliable and better discriminates patients from controls, both versions were used in this study. For the density version, difficulty increases as the density of noise elements increases. For the first 2 staircase reversals, the density changed by 0.2D units (ie, the ratio of the spacing between noise elements and contour elements) per reversal, for the remaining reversals, 0.5D units per reversal. For the jitter version, difficulty increases as the degree of jitter of the contour-defining Gabor elements increases. For the first 2 reversals, the degree of jitter changed by units of 10° per reversal, thereafter, 5° per reversal. In each trial, stimulus was presented for 5 s and participants had to respond within this duration (figure 2).
Fig. 2.
Contour Integration Task: a) density version and b) jitter version. Participants had to locate the contour formed by Gabor elements (left or right side of the screen). a) Difficulty level increases as the density of noise increases. b) Difficulty level increases as the jitter angle of the contour-forming Gabor elements increases. Arrows indicate the location of the contours.
Eye-Contact Perception.
Twenty-six SCZ and 23 HC participants of those who completed the visual integration tasks also completed the eye-contact perception task. Details of this task and results are reported in the study done by Tso et al.3 Briefly, participants made eye-contact judgments (“Looking at me?” yes/no) for faces with varying gaze direction (from averted to direct in ten 10% increments). For each participant, the percentage of “yes” responses was plotted against the continuum of gaze angles, and a logistic function was fitted to the data. Given that the shift of eye-contact perception from “not looking at me” to “looking at me” essentially occurs when eye contact is endorsed 50% of the time, the slope of the logistic curve where “yes” responses is given 50% of the time is therefore used to index how rapid such shift is, with more rapid shifts indicating more dichotomous eye-contact perception and suggesting less uncertainty when making eye-contact judgment. This measure of dichotomous eye-contact perception was found to be a significant predictor of emotional intelligence in SCZ even after taking basic neurocognition into consideration.3 Thus, this measure was used in the analyses in this report, and descriptive statistics for SCZ and HC included in this study are shown in table 1.
Emotional Intelligence.
The Mayer-Salovey-Caruso Emotional Intelligence Test (MSCEIT)25 is a performance-based measure of emotion-related social cognitive skills recommended by the Measurement and Treatment Research to Improve Cognition in Schizophrenia committee.36 Participants completed all 4 parts of the test (perceiving emotion, using emotion, understanding emotion, and managing emotion). Age- and gender-adjusted scores were used in the analyses (see table 1).
Statistical Analyses
For the visual integration tasks, outliers (scores >3 SDs from the mean of the participant’s diagnosis group) were excluded, resulting in the exclusion of 1 SCZ and 1 HC participants from Motion, 1 HC from the Contour-density, and 1 SCZ from Contour-jitter; these were all different individuals. The 2 groups were then compared for thresholds using t tests. The relationships between visual integration and the social cognition measures were examined using correlation and regression analyses.
Results
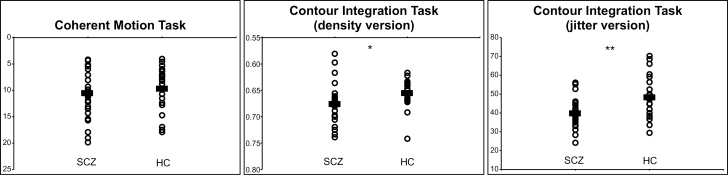
Visual Integration
Thresholds on the visual integration tasks by diagnostic group are displayed in figure 3. For Motion, SCZ (10.63±4.40) showed equal thresholds as HC (9.78±4.15), t(50) = −0.41, P = .68. However, SCZ showed poorer performance than HC on both versions of Contour (density: SCZ = 0.677±0.039, HC = 0.657±0.029, t(49) = −2.10, P = .041; jitter: SCZ = 39.9±7.3, HC = 48.3±11.1, t(49) = −3.23, P = .002).
Fig. 3.
Visual perceptual thresholds of SCZ and HC participants. Horizontal bars indicate group means. For Coherent Motion Task and Contour Integration Task (density version), the y-axis is presented in reverse, so that scores in upper position indicate better performance. *P < .05 and **P < .01.
Relationship With Eye-Contact Perception and Emotional Intelligence
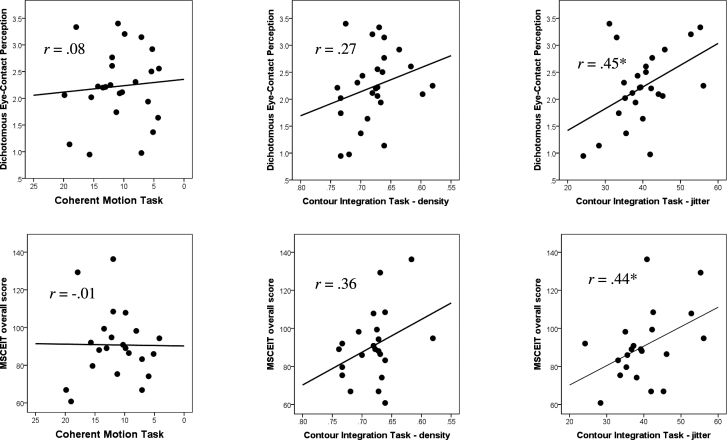
The results of the a priori correlations between visual integration measures and social cognition measures in SCZ are displayed in figure 4. Performance on Motion was not significantly correlated with the social cognition measures. The 2 versions of Contour showed similar positive correlations with dichotomous eye-contact perception and MSCEIT overall score, but only the correlations with the jitter version reached statistical significance.
Fig. 4.
Correlations between visual integration (left: Coherent Motion Task; middle: Contour Integration Task-density; right: Contour Integration Task-jitter) and social cognition measures (top: dichotomous eye-contact perception; bottom: MSCEIT) among SCZ participants. The x-axis for Motion and Contour-density is displayed in reverse, so that values toward the right represent better performance. *P < .05.
Correlations between measures of visual integration, social cognition, and clinical variables among SCZ are shown in online supplementary material 2. Briefly, symptom ratings and chlorpromazine equivalent antipsychotic dose showed virtually no correlations with the performance measures.
Because visual integration as measured with Contour-jitter more strongly discriminated SCZ from HC and showed strong correlations with both social cognition measures, we conducted regression analyses to further analyze the relationship of this early visual processing with higher level social cognition. Among SCZ participants, visual integration explained 19.9% of variance in dichotomous eye-contact perception (F(1, 23) = 5.73, P = .025) and 19.2% of variance in emotional intelligence (MSCEIT overall score) (F(1, 19) = 4.50, P = .047).
When the 2 groups were considered together, diagnosis group accounted for 11.5% of variance in dichotomous eye-contact perception (F(1, 47) = 6.10, P = .017) and 16.7% of variance in emotional intelligence (F(1, 39) = 7.84, P = .008). In separate regression analyses, visual integration accounted for comparable amounts of variance in social cognition: 10.7% in dichotomous eye-contact perception (F(1, 46) = 5.52, P = .023) and 18.2% in emotional intelligence (F(1, 38) = 8.46, P = .006). Visual integration and diagnosis group together accounted for 16.1% of variance in dichotomous eye-contact perception (F(2, 45) = 4.31, P = .019) and 26.3% of variance in emotional intelligence (F(2, 37) = 6.60, P = .004).
Discussion
This study examined visual integration and its relationship with social cognition in schizophrenia. Consistent with our hypothesis and previous findings,12,13 SCZ demonstrated significantly poorer performance than HC on the Contour Integration Task, indicating a reduced ability to integrate individual, local visual features to form a holistic representation. As hypothesized, this perceptual function was significantly correlated with both dichotomous eye-contact perception and the MSCEIT. Additionally, visual integration and the diagnosis of schizophrenia accounted for similar amounts of variance in these 2 social cognitive measures. Taken together, the results are consistent with the hypothesis that deficits in visual integration play a significant role in the impairment in different levels of social information processing in schizophrenia.
The relationship between visual integration and dichotomous eye-contact perception suggests that the compromised ability to efficiently discriminate self-directed vs non-self-directed gaze in schizophrenia may be related to a reduced ability to consider the interrelations of local elements (ie, facial features) when forming judgment of the direction of the eyes. This is consistent with the recent findings that SCZ participants’ eye-contact perception is less affected by contextual factors including head orientation and facial emotion.3 Although holistic perception of faces does not always result in more “accurate” perception of gaze direction per se, as people are poorer at detecting direct gaze from averted than forward faces3,37 and biased to perceiving averted gaze from fearful faces,3,38 it enables the person to process social information in a more functionally adaptive way.
The relationship between visual integration and MSCEIT supported that disruptions in visual integration may have significant implications for higher level socioemotional functioning in schizophrenia. Given this functional implication and its potential sensitivity to treatment effects,39 treatment modalities that can effectively improve visual integration might improve higher level functions like social cognition, as has been demonstrated for early auditory processing and verbal memory.40 Our finding is consistent with cascade models of deficits, wherein higher level functions are impaired as result of early perceptual deficits.8,9 In this study, the variances in both dichotomous eye-contact perception and MSCEIT due to visual integration (Contour-jitter) overlapped with those due to the diagnosis of schizophrenia, suggesting that disrupted data-driven visual perception may account for the observed social cognitive deficits in schizophrenia.
Given the correlational nature of our study, we cannot exclude a role for top-down processing (such as cognitive control) affecting visual integration and social cognition. It should be noted that the amounts of variance in social functional outcomes explained by visual processing deficits in this study and previous structural equation modeling studies8,9 were far less than perfect (<50%). A recent review showed that the difficulty with visual organization for schizophrenia is most pronounced when sophisticated mechanisms and top-down processing are required to process novel, noisy, or highly fragmented forms.19 In fact, a somewhat surprising finding from our correlation results showed that visual integration was correlated with MSCEIT domains that require more sophisticated skills but not the domain of perceiving emotion (see online supplementary material 2). Given the presence of “backward” connections in the visual cortex from the prefrontal/anterior cingulate cortex,41 it is possible that the output of high-level cognitive processes that also govern sophisticated social processes feeds back to influence processing of visual features during contour integration. These altogether suggest that although visual processing deficits may have significant bottom-up effects on higher level functioning in schizophrenia, other mediating/moderating factors and top-down mechanisms must also be considered in order to gain a full understanding of the complex nature of socioemotional deficits in the disorder. In particular, the top-down modulation of input processing in sensory regions by activity in the prefrontal/anterior cingulate cortex that is evident in healthy individuals and in SCZ patients is worth further investigation.28,41,42
This study demonstrated that both Contour-density and Contour-jitter could significantly discriminate SCZ from HC. However, the 2 versions showed only moderate correlation with each other in SCZ (r = .54), suggesting that perceiving a contour against a noisy background reflects a somewhat different ability than perceiving a contour made of elements that do not line up perfectly. This may be because crowding (the disruptive influence of “visual clutter” on contour detection) is more an issue for the density manipulation than the jitter manipulation.43 This difference may account for the differences in correlations with the social cognition variables, especially because schizophrenia patients appear to be more influenced by crowding issues.44 A recent study showed that the jitter manipulation was effective at generating different levels of activation in visual cortical areas known to be sensitive to grouping (ie, V1, V2/V3, and V4).45 Our finding that Contour-jitter appeared to show stronger correlations with the social cognitive measures in SCZ, than did Contour-density, further suggests that the jitter manipulation may be a more effective way to tap into the process of visual integration. However, it should be noted that the clinical and functional correlates of the density version were in the same direction and of similar magnitudes as those of the jitter version, providing converging evidence that visual integration as measured with the Contour Integration Task has important functional implications.
Contrary to a previous study,46 SCZ participants showed intact performance on the Coherent Motion Task. This is possibly due to the methodological differences, including the exclusion of outliers in this study and the use of different paradigm parameters. Some of these parameters (eg, dot velocities and dot density) have been shown to influence the conclusions as to whether individuals with schizophrenia have deficient ability to detect coherent motion46,47 though the findings have been inconsistent and follow-up studies are needed to clarify this issue, particularly in relation to activation in the middle temporal area in the extrastriate cortex, which has been implicated in coherent motion perception.29 Although speculative because of the null results, the finding of limited overlap between Motion and both versions of Contour in this study raises the question whether the Coherent Motion Task is an effective measure of visual integration.
This study was limited by the use of relatively chronic, stable, and medicated outpatients. The limited range of positive and negative symptoms in this sample (table 1) may have limited the power to detect potential correlations between symptoms and the key variables. In addition, because perceptual organization deficits are more pronounced in those who are acutely ill,19 the lack of linkage with symptoms in this study might be due to the low prevalence of disorganization symptoms and clinical stability in this sample. Medication effects were also difficult to isolate though antipsychotic dose was not associated with performance on the visual perception tasks. Results from nonmedicated patients are as yet unknown, but the results of this study can be considered representative of patients in typical psychiatric care settings. Future studies may include other psychiatric groups to address the question whether visual integration deficits and their social functional correlates are specific to schizophrenia. In addition, to rule out a generalized deficit in the relationship between visual integration and higher level social cognition, future studies should develop tasks that can avoid this confound or replicate the results using tasks in which patients perform better than controls due to disrupted visual integration function (eg, the Place and Gilmore task14 and Ebbinghaus illusion).48
To conclude, individuals with schizophrenia showed compromised abilities to integrate individual local visual features into a holistic representation. This perceptual deficit was related to multiple levels of social cognitive deficits in the disorder, calling for further investigations of the dynamic role of visual integration in higher level information processing in the disorder.
Supplementary Material
Supplementary material is available at http:// schizophreniabulletin.oxfordjournals.org.
Funding
University of Michigan, Department of Psychology, Faculty Start-up Grant (to P.J.D.); Barbour Scholar- ship, Mary Malcombson Raphael Fellowship, Rackham Graduate Student Research Grant; American Psycholog- ical Foundation Benton-Meier Neuropsychology Schol- arship (to I.F.T.).
Supplementary Material
Acknowledgments
The authors thank Mei Lun Mui, Chelsey Marsh, and Anita Calwas for their assistance in data collection. The authors reported no biomedical financial interests or potential conflicts of interest relevant to the contact of this manuscript.
References
- 1. Hooker C, Park S. You must be looking at me: the nature of gaze perception in schizophrenia patients. Cogn Neuropsychiatry. 2005;10:327–345 [DOI] [PubMed] [Google Scholar]
- 2. Kohler CG, Walker JB, Martin EA, Healey KM, Moberg PJ. Facial emotion perception in schizophrenia: a meta-analytic review. Schizophr Bull. 2010; 36: 1009–1019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Tso IF, Mui ML, Taylor SF, Deldin PJ. Eye-contact perception in schizophrenia: relationship with symptoms and socioemotional functioning. J Abnorm Psychol. 2012; 121: 616–627 [DOI] [PubMed] [Google Scholar]
- 4. Bora E, Yucel M, Pantelis C. Theory of mind impairment in schizophrenia: meta-analysis. Schizophr Res. 2009; 109: 1–9 [DOI] [PubMed] [Google Scholar]
- 5. Couture SM, Penn DL, Roberts DL. The functional significance of social cognition in schizophrenia: a review. Schizophr Bull. 2006; 32 Suppl 1: S44–S63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Butler PD, Silverstein SM, Dakin SC. Visual perception and its impairment in schizophrenia. Biol Psychiatry. 2008; 64: 40–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Norton D, McBain R, Holt DJ, Ongur D, Chen Y. Association of impaired facial affect recognition with basic facial and visual processing deficits in schizophrenia. Biol Psychiatry. 2009; 65: 1094–1098 [DOI] [PubMed] [Google Scholar]
- 8. Rassovsky Y, Horan WP, Lee J, Sergi MJ, Green MF. Pathways between early visual processing and functional outcome in schizophrenia. Psychol Med. 2011; 41: 487–497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sergi MJ, Rassovsky Y, Nuechterlein KH, Green MF. Social perception as a mediator of the influence of early visual processing on functional status in schizophrenia. Am J Psychiatry. 2006; 163: 448–454 [DOI] [PubMed] [Google Scholar]
- 10. Doniger GM, Foxe JJ, Murray MM, Higgins BA, Javitt DC. Impaired visual object recognition and dorsal/ventral stream interaction in schizophrenia. Arch Gen Psychiatry. 2002; 59: 1011–1020 [DOI] [PubMed] [Google Scholar]
- 11. Kurylo DD, Pasternak R, Silipo G, Javitt DC, Butler PD. Perceptual organization by proximity and similarity in schizophrenia. Schizophr Res. 2007; 95: 205–214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Silverstein SM, Kovács I, Corry R, Valone C. Perceptual organization, the disorganization syndrome, and context processing in chronic schizophrenia. Schizophr Res. 2000; 43: 11–20 [DOI] [PubMed] [Google Scholar]
- 13. Uhlhaas PJ, Phillips WA, Silverstein SM. The course and clinical correlates of dysfunctions in visual perceptual organization in schizophrenia during the remission of psychotic symptoms. Schizophr Res. 2005; 75: 183–192 [DOI] [PubMed] [Google Scholar]
- 14. Place EJ, Gilmore GC. Perceptual organization in schizophrenia. J Abnorm Psychol. 1980; 89: 409–418 [DOI] [PubMed] [Google Scholar]
- 15. Uhlhaas PJ, Phillips WA, Schenkel LS, Silverstein SM. Theory of mind and perceptual context-processing in schizophrenia. Cogn Neuropsychiatry. 2006; 11: 416–436 [DOI] [PubMed] [Google Scholar]
- 16. Schenkel LS, Spaulding WD, Silverstein SM. Poor premorbid social functioning and theory of mind deficit in schizophrenia: evidence of reduced context processing? J Psychiatr Res. 2005; 39: 499–508 [DOI] [PubMed] [Google Scholar]
- 17. Silverstein SM, Schenkel LS, Valone C, Nuernberger SW. Cognitive deficits and psychiatric rehabilitation outcomes in schizophrenia. Psychiatr Q. 1998; 69: 169–191 [DOI] [PubMed] [Google Scholar]
- 18. Gold JM, Barch DM, Carter CS, et al. Clinical, functional, and intertask correlations of measures developed by the Cognitive Neuroscience Test Reliability and Clinical Applications for Schizophrenia Consortium. Schizophr Bull. 2012; 38: 144–152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Silverstein SM, Keane BP. Perceptual organization impairment in schizophrenia and associated brain mechanisms: review of research from 2005 to 2010. Schizophr Bull. 2011; 37: 690–699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Caharel S, Bernard C, Thibaut F, et al. The effects of familiarity and emotional expression on face processing examined by ERPs in patients with schizophrenia. Schizophr Res. 2007; 95: 186–196 [DOI] [PubMed] [Google Scholar]
- 21. Turetsky BI, Kohler CG, Indersmitten T, Bhati MT, Charbonnier D, Gur RC. Facial emotion recognition in schizophrenia: when and why does it go awry? Schizophr Res. 2007;94:253–263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wynn JK, Lee J, Horan WP, Green MF. Using event related potentials to explore stages of facial affect recognition deficits in schizophrenia. Schizophr Bull. 2008; 34: 679–687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Tso IF, Calwas AM, Chun J, Taylor SF, Deldin PJ. Simultaneous processing of gaze direction and facial emotion in schizophrenia: an ERP study. Under revision.
- 24. Emery NJ. The eyes have it: the neuroethology, function and evolution of social gaze. Neurosci Biobehav Rev. 2000; 24: 581–604 [DOI] [PubMed] [Google Scholar]
- 25. Mayer JD, Salovey P, Caruso DR. Mayer-Salovey-Caruso Emotional Intelligence Test. North Tonawanda, NY: Multi-Health Systems Inc; 1999. [Google Scholar]
- 26. Eack SM, Greeno CG, Pogue-Geile MF, Newhill CE, Hogarty GE, Keshavan MS. Assessing social-cognitive deficits in schizophrenia with the Mayer-Salovey-Caruso Emotional Intelligence Test. Schizophr Bull. 2010; 36: 370–380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kee KS, Horan WP, Salovey P, et al. Emotional intelligence in schizophrenia. Schizophr Res. 2009; 107: 61–68 [DOI] [PubMed] [Google Scholar]
- 28. Taylor SF, Chen AC, Tso IF, Liberzon I, Welsh RC. Social appraisal in chronic psychosis: role of medial frontal and occipital networks. J Psychiatr Res. 2011; 45: 526–538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Butler PD, Chen Y, Ford JM, Geyer MA, Silverstein SM, Green MF. Perceptual measurement in schizophrenia: promising electrophysiology and neuroimaging paradigms from CNTRICS. Schizophr Bull. 2012; 38: 81–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. First MB, Spitzer RL, Gibbon M, Williams JB. Structured Clinical Interview for DSM-IV Axis I Disorders, Patient Edition (SCID-P), version 2.0. New York: Biometrics Research; 1995. [Google Scholar]
- 31. Andreasen NC. The Scale for the Assessment of Positive Symptoms (SAPS). Iowa City, IA: University of Iowa; 1984
- 32. Andreasen NC. Scale for the Assessment of Negative Symptoms (SANS). Iowa City, IA: University of Iowa; 1983
- 33. Field DJ, Hayes A, Hess RF. Contour integration by the human visual system: evidence for a local “association field”. Vision Res. 1993; 33: 173–193 [DOI] [PubMed] [Google Scholar]
- 34. Silverstein SM, Kovács I, Corry R, Valone C. Perceptual organization, the disorganization syndrome, and context processing in chronic schizophrenia. Schizophr Res. 2000; 43: 11–20 [DOI] [PubMed] [Google Scholar]
- 35. Silverstein SM, Keane BP, Barch DM, et al. Optimization and validation of a visual integration test for schizophrenia research. Schizophr Bull. 2012; 38: 125–134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Green MF, Olivier B, Crawley JN, Penn DL, Silverstein S. Social cognition in schizophrenia: recommendations from the measurement and treatment research to improve cognition in schizophrenia new approaches conference. Schizophr Bull. 2005; 31: 882–887 [DOI] [PubMed] [Google Scholar]
- 37. Itier RJ, Alain C, Kovacevic N, McIntosh AR. Explicit versus implicit gaze processing assessed by ERPs. Brain Res. 2007; 1177: 79–89 [DOI] [PubMed] [Google Scholar]
- 38. Tipples J. Fear and fearfulness potentiate automatic orienting to eye gaze Cogn Emot. 2006; 20: 309–320 [Google Scholar]
- 39. Silverstein SM, Keane BP. Perceptual organization in schizophrenia: plasticity and state related change. Learn Percept. 2009; 1: 229–261 [Google Scholar]
- 40. Fisher M, Holland C, Merzenich MM, Vinogradov S. Using neuroplasticity-based auditory training to improve verbal memory in schizophrenia. Am J Psychiatry. 2009; 166: 805–811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Sarter M, Gehring WJ, Kozak R. More attention must be paid: the neurobiology of attentional effort. Brain Res Rev. 2006; 51: 145–160 [DOI] [PubMed] [Google Scholar]
- 42. Sarter M, Lustig C, Taylor SF. Cholinergic contributions to the cognitive symptoms of schizophrenia and the viability of cholinergic treatments. Neuropharmacology. 2012; 62: 1544–1553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Robol V, Casco C, Dakin SC. The role of crowding in contextual influences on contour integration. J Vis. 2012; 12: 3 [DOI] [PubMed] [Google Scholar]
- 44. Kraehenmann R, Vollenweider FX, Seifritz E, Kometer M. Crowding deficits in the visual periphery of schizophrenia patients. PLoS One. 2012; 7: e45884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Silverstein SM, Berten S, Essex B, Kovács I, Susmaras T, Little DM. An fMRI examination of visual integration in schizophrenia. J Integr Neurosci. 2009; 8: 175–202 [DOI] [PubMed] [Google Scholar]
- 46. Chen Y, Nakayama K, Levy D, Matthysse S, Holzman P. Processing of global, but not local, motion direction is deficient in schizophrenia. Schizophr Res. 2003;61:215–227 [DOI] [PubMed] [Google Scholar]
- 47. Slaghuis WL, Holthouse T, Hawkes A, Bruno R. Eye movement and visual motion perception in schizophrenia II: global coherent motion as a function of target velocity and stimulus density. Exp Brain Res. 2007; 182: 415–426 [DOI] [PubMed] [Google Scholar]
- 48. Uhlhaas PJ, Phillips WA, Mitchell G, Silverstein SM. Perceptual grouping in disorganized schizophrenia. Psychiatry Res. 2006; 145: 105–117 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.