Figure 3.
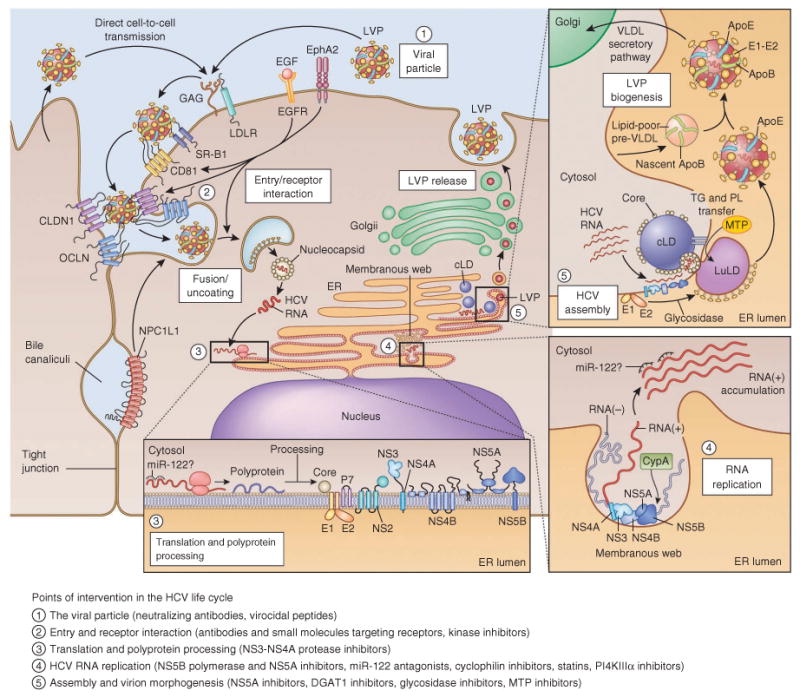
The HCV life cycle and points of intervention. Points of intervention in the HCV life cycle are marked with numbered circles, and types of inhibitors of the individual steps are indicated in the legend. Interaction of extracellular HCV LVPs (1) with cellular surface receptors initiates the entry process (2), which can also occur from direct cell-to-cell transmission. After pH-dependent fusion and uncoating, the incoming HCV genome is translated and the resulting polyprotein processed (bottom inset and Fig. 2, (3)). Replication takes place in ER-derived membrane spherules (membranous web, bottom right inset, (4)), the architecture of which remains to be fully defined76. The spatiotemporal contribution of miR-122 binding to the HCV genome is not yet fully understood, and miR-122 presence is indicated with ‘?’. In the assembly and release process (top right inset, (5)), core protein is transferred from cLDs to form nucleocapsids that, assisted by NS5A, are loaded with RNA. Replicase proteins supposedly bind HCV RNA during transfer from replication to packaging, the intracellular sites of which might converge. It is not clear whether the RNA is transiently located on the cLD. The p7, NS2 and NS3-NS4A proteins are also involved in coordination of assembly. HCV virion morphogenesis is coupled to the VLDL pathway, and particles are produced as LVPs. Particles released from cell culture have less ApoB association and resemble the ApoB-deficient particle illustrated. LVPs also associate with ApoC, which for simplicity is not shown here. EphA2, ephrin receptor type A2; GAG, glycosaminoglycans; PL, phospholipids; TG, triglycerides. In the translation inset, NS5A is shown as a dimer, though several other HCV proteins also form homo- or heterodimers or oligomers. For comprehensive discussion and illustration of HCV assembly and release and of membrane-associated HCV protein structures, see refs. 25,167, respectively.
