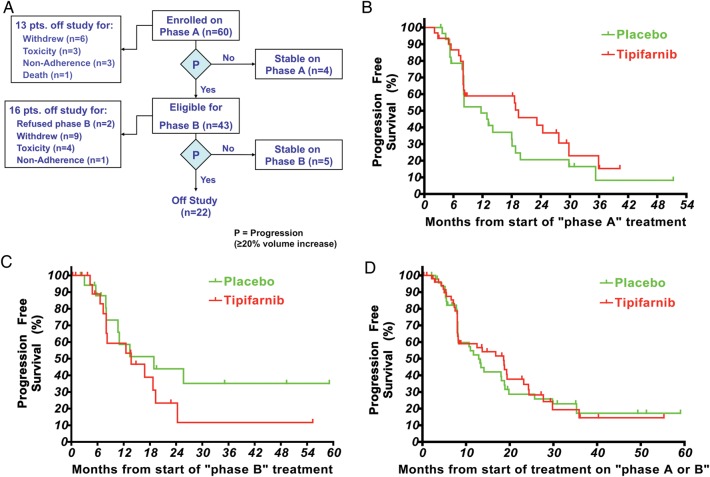
Fig. 1.
(A) Trial status of eligible participants at the time of trial unblinding. (B) Progression-free survival on phase A. The median time to progression for tipifarnib (n = 31) was 19.2 months and for placebo (n = 29) 10.6 months (1-tailed P = .12). Participants who were removed from study for reasons other than progression had their follow-up censored at the times shown with tick marks. (C) Progression-free survival on phase B. The median time to progression for tipifarnib (n = 22) was 13.3 months and for placebo (n = 18) 14.5 months (1-tailed P = .14). Participants who were removed from the study for reasons other than progression had their follow-up censored at the times shown with tick marks. (D) Progression-free survival for all participants who received placebo or tipifarnib irrespective of the treatment phase. The median time to progression for placebo (n = 47) was 13.0 months and for tipifarnib (n = 53) 18.2 months. Forty participants are represented on both curves. Participants who were removed from the study for reasons other than progression had their follow-up censored at the times shown with tick marks.