Table 2.
Scope of the Catalytic, Asymmetric Borylation of α,β-Unsaturated Amides with BBA.

| |
|---|---|
| entry | product[a] |
| 1 |
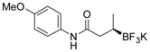 2a 90%, 93:7 er (85%, 93:7 er)[b] |
| 2 |
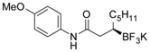 2b 82%, 97:3 er |
| 3 |
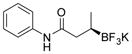 2c 80%, 92:8 er |
| 4 |
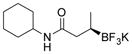 2d 82%, 95:5 er (71%, 1.5 equiv BBA)[c] |
| 5 |
2e 51%, 93:7 er |
| 6 |
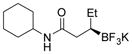 2f 86%, 98:2 er |
| 7 |
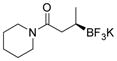 2g 82%, 92:8 er |
| 8 |
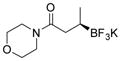 2h 84%, 97:3 er |
| 9 |
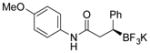 2i 92%, 96:4 er |
| 10 |
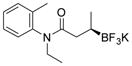 2j 71%, 76:24 er |
| 11 |
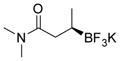 2k 86%, 70:30 er |
| 12 |
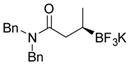 2l 80%, 66:34 er |
| 13 |
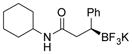 2m 77%, 72:28 er |
| 14 |
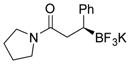 2n 85%, 78:22 er |
Enantiomeric excess was determined by oxidation of 2 followed by SFC analysis of the corresponding alcohol.
Reaction performed on a 5.5 mmol scale with 2.5 mol % Cu(MeCN)4PF6, 2.5 mol % L2.
Reaction performed on a 3 mmol scale.
