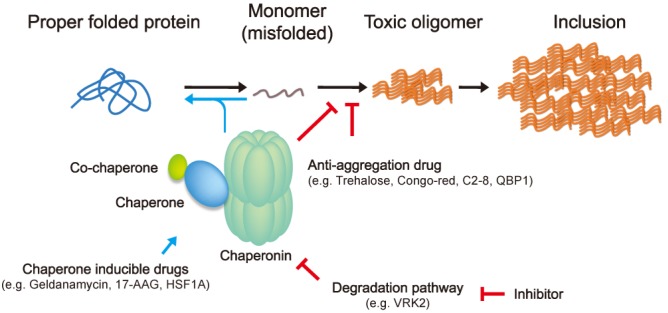
Fig. 2.
Therapeutic approach to inhibit mHtt aggregation. An initiating event in aggregation may conversion of mutant huntingtin to an abnormal conformation. It leads to progress through oligomeric intermediates to the formation of large aggregates. Although there are still controversies which one is more toxic between soluble oligomer and insoluble aggregates, inhibition early in the aggregation pathway would be beneficial to the cells because it may prevent the formation of putative toxic insults. Accordingly, molecular chaperones and anti-aggregation drugs are shed the light in this therapeutic intervention. Particularly, molecular chaperones not only induce proper folding of misfolded proteins by interacting with exposed hydrophobic surfaces, but also inhibit aggregation with mutant huntingtin itself. A number of HSF1 activating drugs have been developed to induce the Heat-shock proteins, but transcription of TRiC/CCT was not affected by HSF1. Thus, another pathway is necessary to modulate the TRiC/CCT levels such as inhibition of degradation pathway. Recently, one report reveals that VRK2 facilitates the TRiC/CCT protein degradation through increase of its ubiquitination. 17-allylamino-17-demethoxygeldanamycin (17-AAG), polyglutamine binding peptide 1 (QBP1), vaccinia-related kinase 2 (VRK2).