Figure 1.
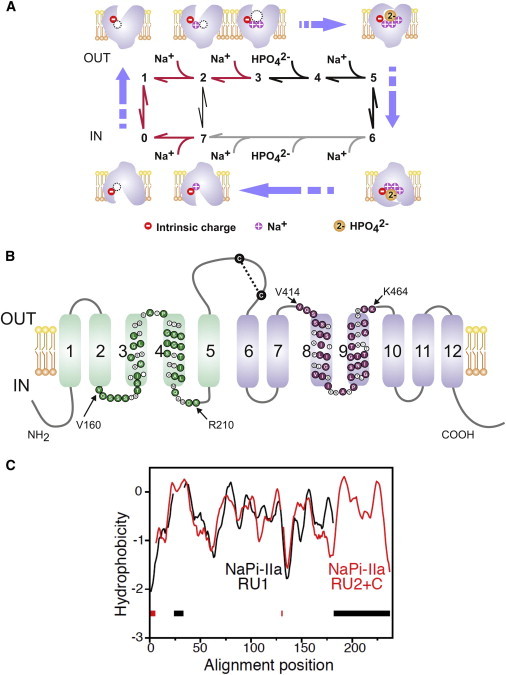
Kinetic and structural features of NaPi-IIa. (A) The transport cycle is depicted as a sequence of partial reactions between conformational states, numbered 0–7 (reviewed in (12)). Cartoons illustrate the ordered nature of protein-substrate interaction based on experimental evidence. Partial reactions on the cytosolic side have not been explicitly identified except the last Na+ release (transition 7 → 0) (68). Red arrows: electrogenic partial reactions (involving charge movement); black arrows: electroneutral partial reactions. In the absence of external Pi, Na+ ions can also translocate via a leak transition (2 ↔ 7) (66). For the electroneutral cycle (of NaPi-IIc), all transitions are electroneutral and the first Na+ ion to bind (transition 1 → 2) is hypothesized not to translocate (58). (B) Secondary topology of NaPi-IIa based on previous experimental evidence and bioinformatic predictions (12). The assumed boundaries of repeat regions (rat sequence) are indicated (52); colored symbols indicate identical or conserved residues in each repeat. A disulfide bridge links the two halves of the protein in the large extracellular loop. (C) Hydropathy plots of the regions predicted to contain the structural repeats in NaPi-II, averaged over a set of sequence homologs and aligned using AlignMe. Region 1, containing residues 86–256 of the human NaPi-IIa (RU1, black) is aligned to region 2 plus C-terminus consisting of residues 335–564 (RU2+C, red). Gaps in the alignment are indicated by dashes along the base of the plot. To see this figure in color, go online.
