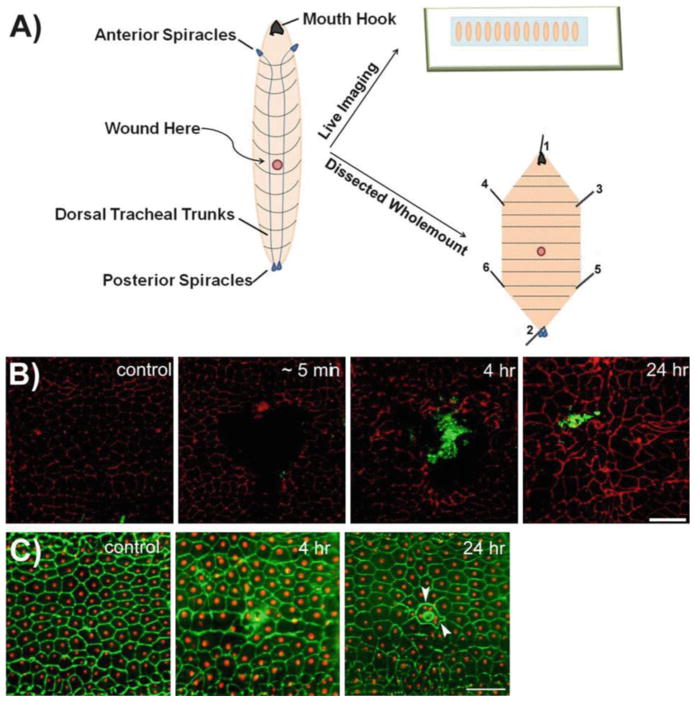
Fig. 1.
Two methods for epidermal wounding of Drosophila larvae. (a) Schematic representation of wounding and mounting of Drosophila larvae. The left illustration depicts the dorsal side of the Drosophila larvae, where the dorsal tracheal trunks connecting the anterior and posterior spiracles are clearly visible. Typically, the A4 segment of the Drosophila larva is chosen for wounding. The epidermal wounds can be visualized either by live imaging (upper right ) when reporter lines are used or by dissecting the larval epidermis and immunostaining for appropriate markers (lower right ). Numbers on dissected larva indicate the order in which pins should be inserted during dissections (see Subheading 3.6). (b) Dissected epidermal whole mounts of control or pinch-wounded larvae stained for Fasciclin III (red) at the indicated times after wounding. Larvae are of genotype w;;Pxn-Gal4, UAS-GFP/+. The GFP-expressing hemocytes are stained with anti-GFP (green ) antibody and the rapid accumulation of hemocytes at the wound site can be seen. At 24 h after wounding, the wound gap is completely closed as observed through Fasciclin III staining. Scale bar 100 μm (adapted from Babcock et al. [17], Copyright © 2008 by The National Academy of Sciences of the USA). (c) Dissected epidermal whole mounts of control or puncture-wounded w; e22c-Gal4, UAS DsRed2-Nuc/+ larvae stained with anti-Fasciclin III (green). Scab formation and epidermal cell–cell fusion around the scab (arrowhead ) can be seen. Scale bar 100 μm